Introduction
Major Depressive Disorder (MDD) is a significant public health issue, affecting around 3.8% of the global population. This translates to approximately 300 million people according to the World Health Organization (WHO). In Spain, the prevalence is even higher, with 6.68% of the population affected, according to the Spanish National Health Survey conducted in 2017. MDD is not just a growing health problem but also a major socioeconomic burden. In 2017, the overall annual costs associated with depression in Spain were estimated at €6145 million. However, 67% of these costs attributed to indirect expenses like loss of productivity.
Current Treatment Approaches and Their Limitations
The standard treatment for MDD involves psychotherapy and pharmacotherapy with antidepressant medications (ADMs). Often, adjuvants such as antipsychotics and mood stabilisers are added. Treatment decisions are often made on a case-by-case basis, involving shared decision-making between patients and practitioners. This flexibility, however, often leads to inconsistent treatment choices, resulting in high failure rates of up to 75%.
Typically, 6 to 8 weeks of adequate dosage are required to assess ADM effectiveness. If the chosen ADM is ineffective, guidelines recommend switching to a different ADM and starting anew (American Psychological Association, 2019). This delay in response leads to low compliance, treatment failure, and increased healthcare costs. Each failed ADM attempt not only delays remission but also increases both direct healthcare costs and indirect costs related to productivity loss. After two failed ADM attempts, MDD is commonly referred to as treatment-resistant depression (TRD), which can increase costs by up to 40%.
The Promise of Pharmacogenetic Screening
Pharmacogenetic (PGx) screening can potentially mitigate these issues by guiding ADM choices based on individual genetic profiles. The Clinical Pharmacogenetics Implementation Consortium (CPIC) recommends pharmacogenetic (PGx) screening for the CYP2C19 and CYP2D6 genes, which metabolise most tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs). These genes are classified by their metaboliser phenotypes: ultrarapid, rapid, normal, intermediate, and poor.
Up to 30% of MDD patients may have unfavourable metaboliser phenotypes, leading to medication trial failures. Randomised controlled trials, such as the PRIME Care trial and the Canadian Genomic Applications Partnership Program – MDD, have shown the usefulness of PGx-guided ADM choices in MDD.
Cost-Effectiveness Analysis
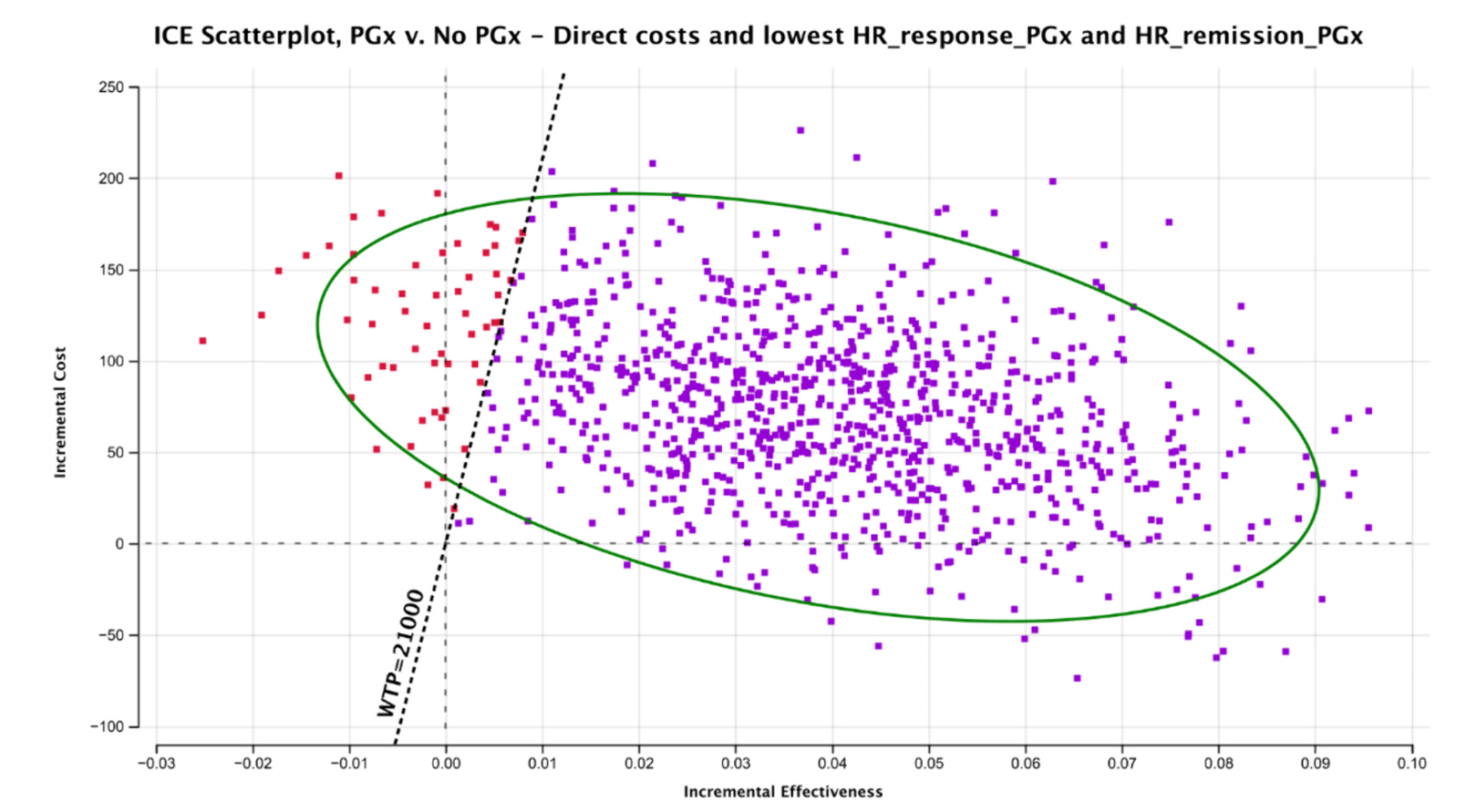
Studies consistently show that PGx screening is a cost-effective strategy. A meta-analysis reported pooled risk ratios for PGx-guided versus unguided ADM treatment: 1.36 for response and 1.74 for remission. The results of a recent analysis suggest that implementing PGx screening to guide treatment choices in MDD would be a cost-effective strategy in the Spanish National Health System (SNHS). The negative Incremental Cost-Effectiveness Ratio (ICER) indicates that the PGx strategy would save money and improve quality-adjusted life years (QALYs). Even in the worst-case scenario, the ICER had a 94.3% probability of cost-effectiveness at the €21,000/QALY threshold.
Other papers have also reported that PGx-guided ADM choice is cost-effective, although expected costs and rewards vary. Grossl et al found that PGx testing saved $2918 in direct medical costs and $1680 in indirect costs over three years, while also yielding 2.07 QALYs compared to 1.97 QALYs without PGx testing. Carta et al built two separate Markov models to evaluate PGx screening of CYP2C19 and CYP2D6, finding this strategy cost-effective at a high willingness to pay (WTP) threshold of €75,000/QALY.
Discussion and Limitations
The primary challenge lies in the variability of PGx screening techniques and the inherent limitations of cost-effectiveness modelling. However, the potential benefits, including reduced treatment failures and indirect costs, make a compelling case for implementing PGx screening in MDD treatment. As the costs of PGx testing decrease, this strategy will become more accessible, further aiding its adoption in clinical practice.
The Spanish model also has its limitations. It did not consider the cost of premature death caused by MDD or TRD. Indirect costs make up two-thirds of the global economic burden of MDD, and TRD patients exhibit higher mortality rates than non-TRD patients. The model also did not contemplate combined therapies or the addition of antipsychotics. It did not consider therapies like esketamine or electroconvulsive therapy (ECT). Future analyses should include these therapies if sufficient data are available.
Conclusion
The results align with the positive reports about the cost-effectiveness of PGx in the literature. Considering these results, the study from Spain affirms that PGx screening in MDD patients is a dominant strategy that would positively impact the SNHS and should be implemented in everyday clinical practice. The gradual reduction in PGx testing costs will make this strategy more accessible and aid its implementation in the SNHS.
