
Introduction
Human papillomavirus (HPV) vaccination is crucial for preventing cervical cancer. The World Health Organization (WHO) has set a target for 90% HPV vaccination coverage by 2030. Despite this, Japan faces significant challenges in achieving this goal. A recent published study explores the complexities surrounding HPV vaccination coverage in Japan, focusing on historical context, current statistics, and future strategies.
Historical Context and Current Challenges
In 2010, Japan introduced a public subsidy program for HPV vaccination targeting female students in grades 7 to 11. By 2013, the HPV vaccine was included in Japan’s national routine vaccination program for girls in grades 6 to 10. However, media reports about severe side effects led to a temporary suspension of the government’s proactive recommendation. This resulted in a dramatic decline in public trust and vaccination rates.
WHO’s Criticism and Public Perception
In 2015, the WHO criticised Japan’s suspension of the HPV vaccine recommendation. They warned that policy decisions based on weak evidence could cause real harm. Despite the vaccine’s proven efficacy in preventing HPV-related cancers, public concern continued to grow, further decreasing vaccination rates.
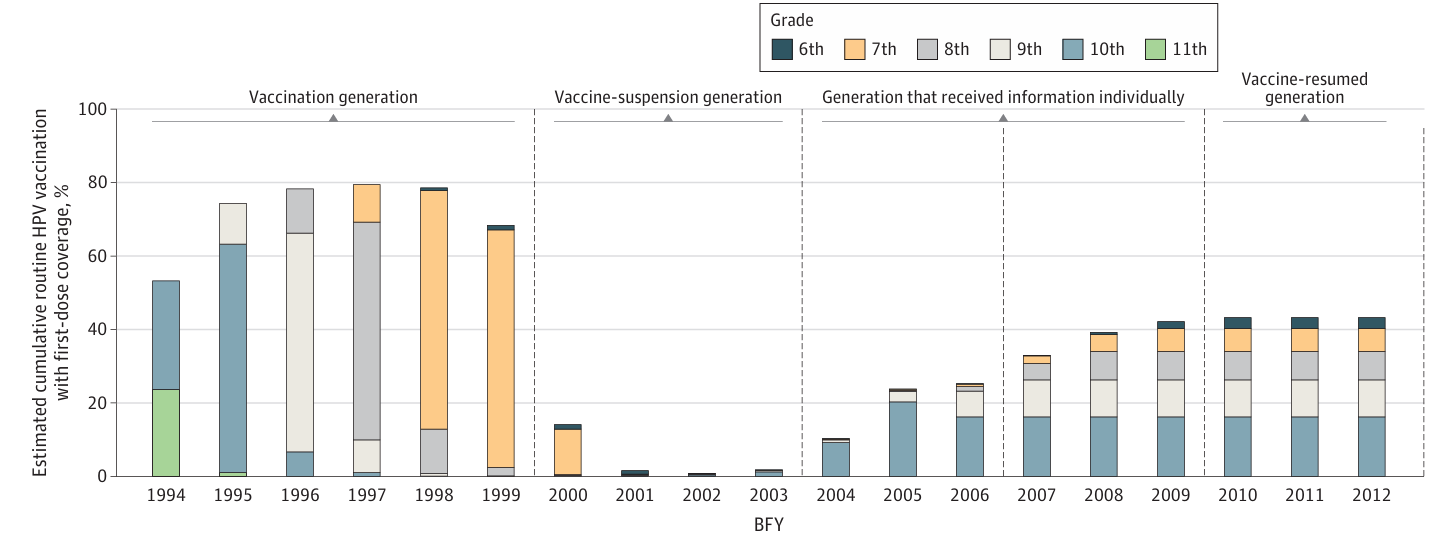
Vaccination Coverage by Birth Year
Yagi et al., calculated birth year-specific vaccination coverage in Japan. The findings showed that HPV vaccination rates among Japanese women remained low. Even with proactive recommendations resuming in 2022, the cumulative first-dose coverage is projected to fall short of the WHO’s 90% target by 2028.
In contrast, other childhood vaccines in Japan have high coverage rates. For instance, the Haemophilus influenzae type b vaccine had a coverage rate of 95.9% in 2017-2018. Similarly, the COVID-19 vaccine had a 66.9% coverage rate among 12- to 19-year-olds. These statistics highlight the unique challenges faced by the HPV vaccination program.
Learning from Other Countries
Countries like Ireland have successfully improved HPV vaccination rates after experiencing declines. In Ireland, a collaborative effort involving 35 organisations led to a 10% increase in vaccination coverage within a year. Japan could adopt a similar multi-stakeholder approach to address its HPV vaccination challenges.
The Ministry of Health, Labour, and Welfare (MHLW) should recognise the HPV vaccination situation as a public health crisis. A nationwide campaign, similar to Ireland’s, could help restore public trust. Furthermore, establishing a comprehensive HPV vaccination database would facilitate better monitoring and targeted interventions.
Expanding Vaccination Programs
To improve HPV vaccination coverage, Japan should consider extending subsidised vaccinations to teenage boys. Strengthening cervical cancer screening recommendations and introducing HPV testing are also crucial steps. These measures would help mitigate the long-term impact of low vaccination rates.
Addressing Vaccine Hesitancy
Overcoming public hesitancy towards the HPV vaccine is essential. Educational campaigns should focus on the vaccine’s safety and efficacy, supported by data from both domestic and international studies. Engaging healthcare providers in these efforts would further enhance public confidence.
Conclusion
Japan faces significant challenges in achieving the WHO’s HPV vaccination coverage targets. Despite resuming proactive recommendations, vaccination rates remain low. Immediate and robust measures are needed to improve public trust and vaccination uptake. By learning from other countries and implementing comprehensive strategies, Japan can make significant strides towards better public health outcomes.
