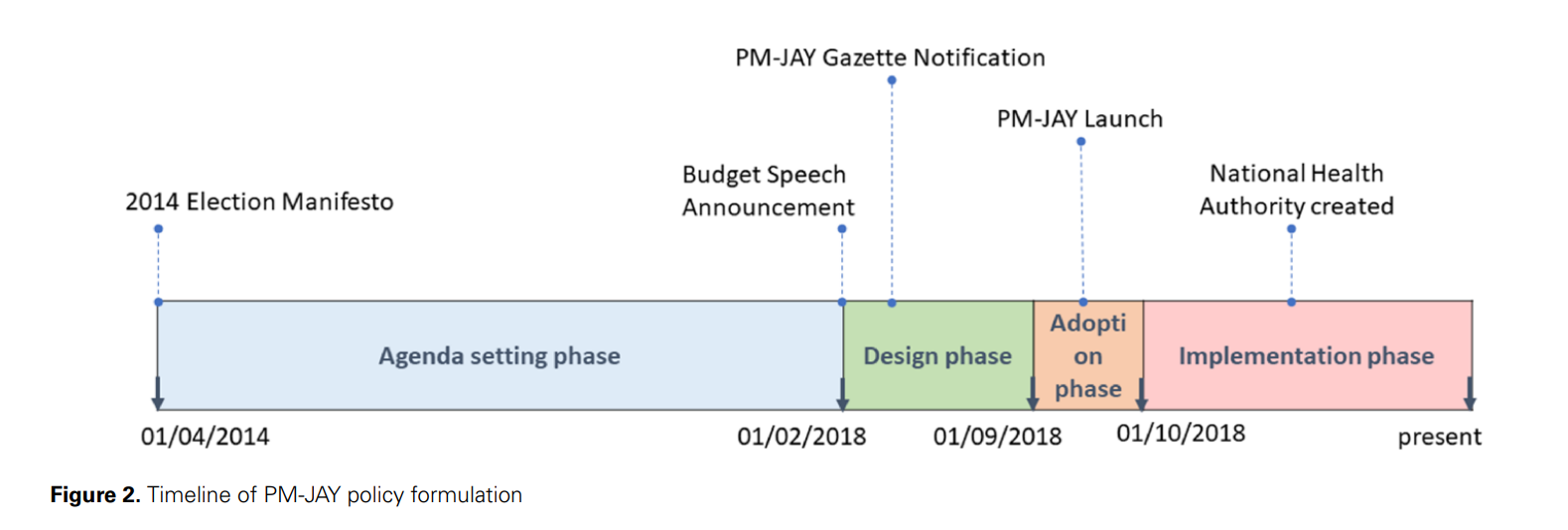
The PM-JAY health insurance scheme, launched in India in 2018, was a crucial step towards universal health coverage (UHC). The policy’s design was shaped by a mix of technical and political elements, with a focus on bureaucratic aspects rather than ideological ones. The scheme was introduced by the ruling government just before national elections, leveraging policy legacies from previous and state insurance schemes. Key decisions such as the coverage amount and portability of benefits were centrally driven, while inputs from Indian states influenced features like the mode of implementation and provider network. This balanced approach facilitated the adoption of the reform. However, the scheme’s success hinges on addressing systemic issues and involving all stakeholders. As PM-JAY continues to be implemented, understanding the politics, power dynamics, and structural issues that shaped its formulation will be crucial.