
How the 340B Program Came Into Existence
The 340B Drug Pricing Program was formed in 1992 with the intention of providing financial assistance to hospitals and clinics that are part of the safety net and serve populations that are underserved or have low incomes. The Medicaid Drug Rebate Program underwent certain revisions, which resulted in the birth of this program, which provides patients with discounts on the prices of outpatient medications. These developments led to pharmaceutical companies discontinuing direct discounts on medicines. This change strained the budgets of hospitals and clinics.
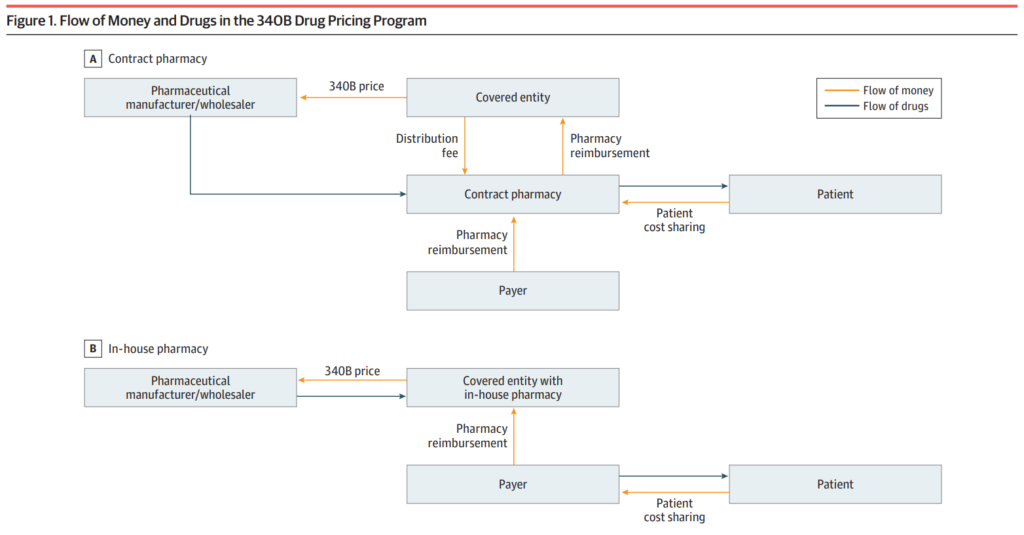
The Importance of the Program and Its Role in It
The 340B program mandates that pharmaceutical companies that take part in Medicaid offer eligible medical facilities and clinics, often known as “covered entities,” access to their products at reduced pricing. After that, these organisations are in a position to charge non-discounted pricing to all payers, so generating revenue that can be put toward the payment of healthcare services and operations.
Recent studies show that the 340B program has significantly benefited covered companies, pharmacies, and patients since its inception. This has led to substantial growth in the program. The covered entities have utilised the program’s funds to offset uncompensated care costs and staff wages. They have also expanded healthcare services and programs, providing pharmaceuticals to patients at reduced prices.
Pharmaceutical manufacturers have seen a revenue decline due to the 340B program. This is why they have initiated several legal challenges against the program. The program now carries a perception of legal uncertainty. This uncertainty stems from ongoing legal disputes and varying court verdicts.
Nevertheless, this research also highlighted several areas of the 340B program that could benefit from potential future changes. It is difficult to investigate these activities and analyse the benefits they have because, for instance, there are currently no regulations on how covered organisations should spend their 340B revenue. It is absolutely essential to have extra data reporting and control as the program continues to expand, bringing in a greater number of covered companies and serving a greater number of patients.
