
UK government funding for dementia research has declined from its peak in 2018-2019. Despite this, the field shows high potential for research in the coming years. By 2040, over 1.2 million people in the UK are expected to have dementia, a 30% increase from 2022. The economic benefits of dementia research can be measured through an economic impact assessment (EIA), which considers various dimensions of dementia research. The report also highlights the significant impact of COVID-19 on people with dementia.
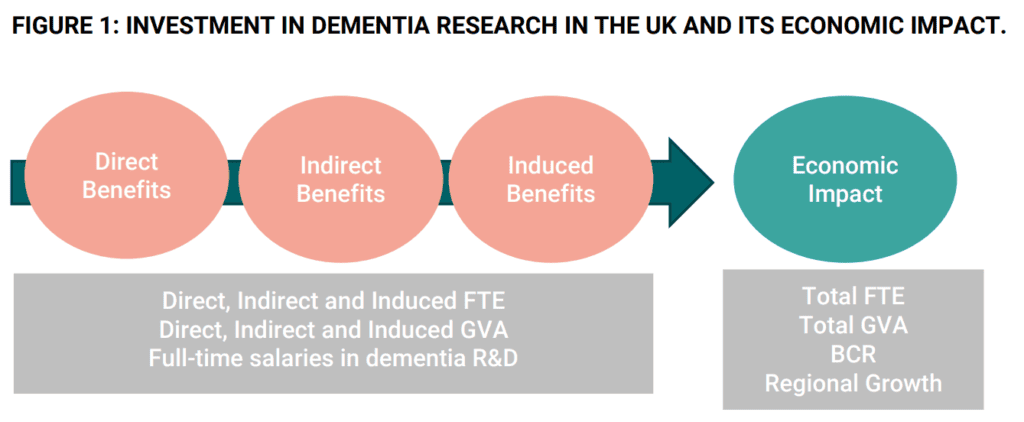
- Dementia research in 2019/20 generated 7,353 full-time equivalent (FTE) jobs and contributed over £529 million in gross value added (GVA) to the UK economy.
- Directly, dementia research supported 2,607 FTE jobs, including 2,059 research/scientific and technical roles and 548 administrative roles, contributing over £276 million in GVA.
- Indirectly, dementia research supported and created 4,746 FTE jobs, contributing over £252 million in GVA.
- Full-time salaries in dementia R&D and administration are, on average, 41% and 24.5% higher respectively than the average salary across all jobs in a region.
The risk of developing dementia or becoming an informal carer for a person with dementia is high. The study found that every £1 invested in dementia research generated £2.59 of economic benefit in the UK during 2019/20, and this is expected to increase to £2.91 on average in the next 20 years. Thus, increased investment in dementia research could improve the lives of those affected by dementia and create long-term economic growth in the UK.