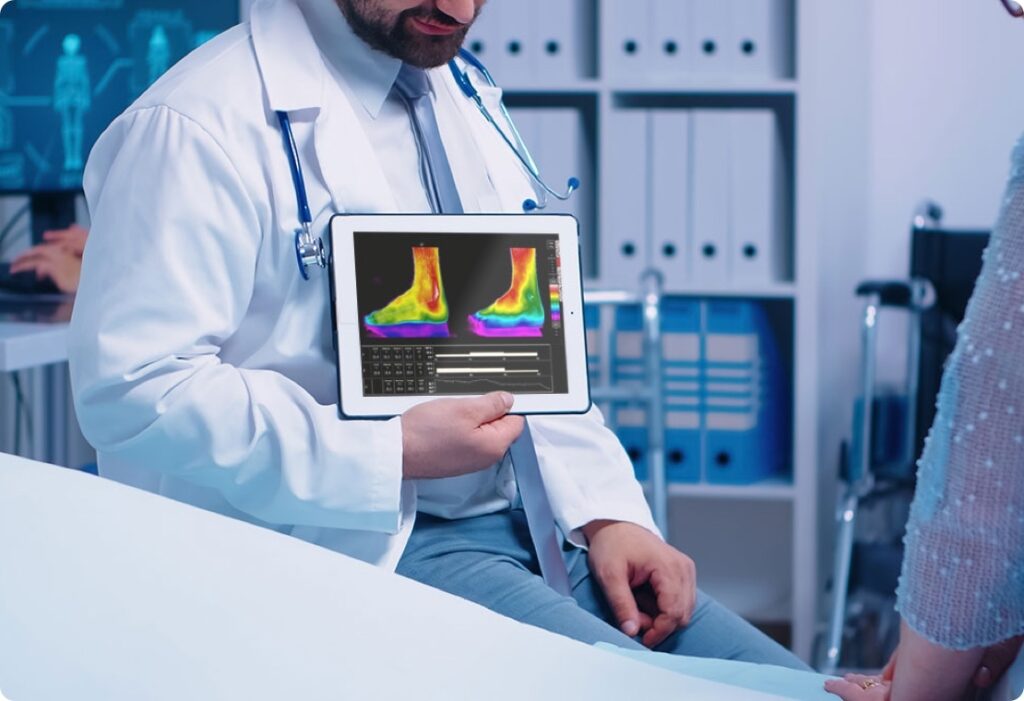
FinCCHTA, the Finnish Coordinating Centre for Health Technology Assessment, has recommended the use of Thermidas, a non-invasive thermal imaging system, to support diagnostics and monitor treatment. The system, which includes imaging analysis and reporting software, is particularly useful for measuring skin surface temperature changes.
Thermidas has been shown to be effective in monitoring temperature differences, indicating changes in the circulatory index, subclinical infections, the severity of vascular disease, and specific complications of Charcot neuro-osteoarthropathy. It has also been found to be a reliable and reproducible method for measuring temperature across areas, compared to traditional point-like temperature measurements in healthy adults.
The system has been particularly beneficial in the monitoring of diabetic foot ulcers and rheumatoid arthritis. A study demonstrated that infrared thermal imaging was able to detect temperature differences in the feet of a group of patients at high risk due to diabetes. Another cross-sectional study showed that combined thermal and ultrasound imaging provides better information about the area under examination than either imaging method used alone in assessing arthritis in rheumatoid arthritis cases.
The Thermidas system is recommended for use until April 2026. The manufacturer has provided evidence of theoretical cost savings, particularly in the monitoring of diabetic podiatric conditions and inflammatory rheumatoid arthritis, as well as in support of diagnostics. The purchase price of the system includes deployment training, with post-acquisition product and maintenance support subject to a fee.