
The Ongoing Issue of Unapproved Fixed-Dose Combination Drugs in India
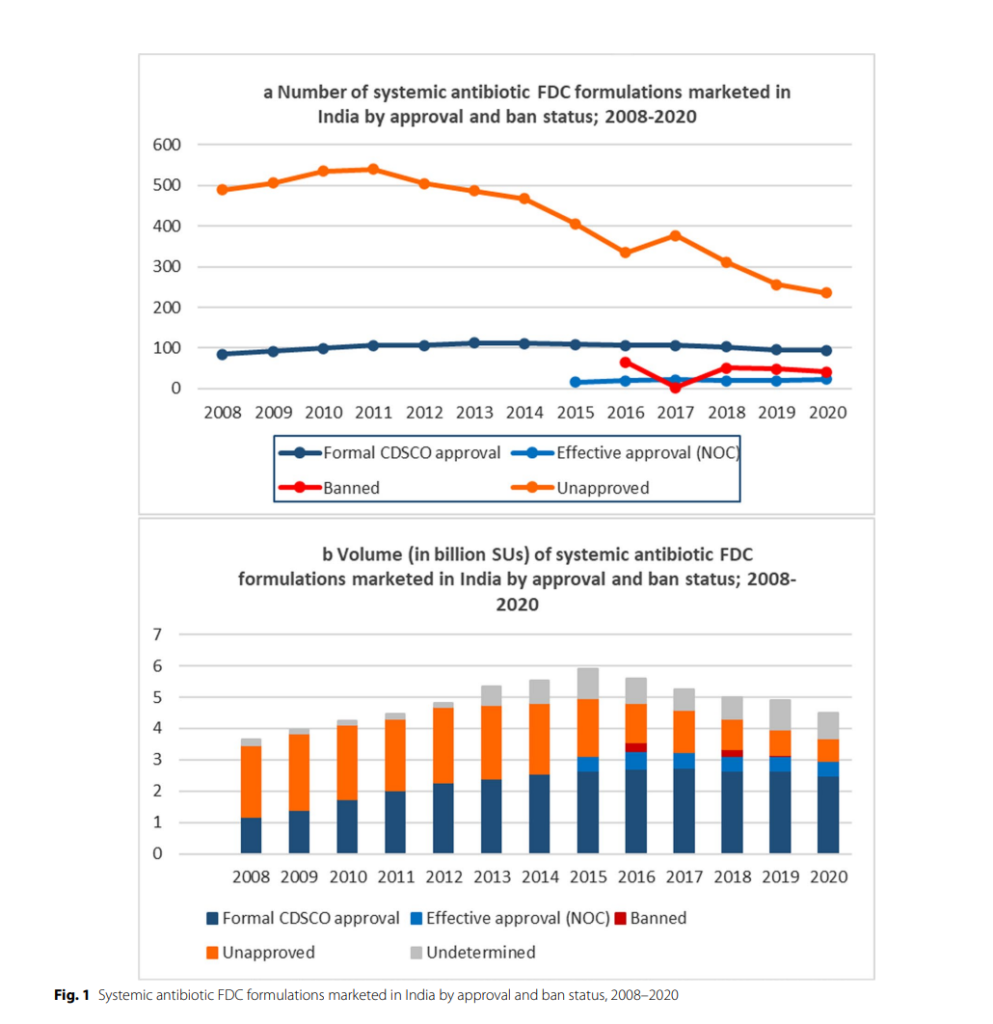
The regulation of medicines in India, a responsibility shared between the central government and individual states, has been a source of friction and calls for reform. One area of notable concern has been the regulation of Fixed-Dose Combination drugs (FDCs), specifically antibiotic FDCs. Despite regulatory initiatives since 2007, hundreds of unapproved antibiotic FDC formulations remained on the Indian market, Accounting for over 700 million of the 4.5 billion standard units sold in 2020. This issue extends beyond India’s borders, contributing to the global concern of increasing antimicrobial resistance.
The Impact on Public Health and Global Concerns
India’s vast sales of centrally unapproved FDCs pose a significant public health concern. This concern escalates when considering the context of increasing antimicrobial resistance. In 2020, 41.5% of the 4.5 billion standard units of antibiotic FDCs sold were attributed to combinations listed as ‘not recommended’ by WHO. The limited progress in controlling these FDCs is a result of weak, convoluted, and inefficient regulatory enforcement.
Recommendations and Further Steps
Regular surveillance of the market, informed regulatory enforcement, and antimicrobial stewardship are recommended. Criminal proceedings should be considered against manufacturers of the formulations marketed without a record of prior Central Drugs Standard Control Organisation (CDSCO) approval. The prescription of only well-evidenced FDCs, such as those listed in National List of Essential Medicines (NLEM), would reduce the use of FDCs and facilitate antimicrobial stewardship. Further research is needed on the control measures and trends in sales of FDCs in other therapeutic areas.
