Introduction
The role of real-world evidence (RWE) in the Centers for Medicare & Medicaid Services (CMS) price negotiations is becoming increasingly significant. As CMS embark on drug price negotiations, the integration of RWE is crucial. This article explored the importance of RWE, the challenges faced, and recommendations for a robust framework.
The Importance of Real-World Evidence
Randomised controlled trials (RCTs) are the gold standard for assessing treatment efficacy and safety. However, they often exclude patients who represent the broader population. This is where RWE comes into play. RWE, derived from real-world data (RWD) such as claims and electronic health records (EHRs), provides a comprehensive view of a drug’s performance in everyday clinical practice.
RWE is particularly valuable for CMS as it considers drugs that have been on the market for at least seven years. This allows sufficient time for RWD to accumulate, offering insights into the drug’s effectiveness in various patient populations. For instance, a study found that RWE can help understand therapeutic alternatives and unmet needs, which are critical for CMS’s price-setting decisions.
Health Technology Assessment and RWE
Health technology assessment (HTA) agencies, such as NICE in the UK, have long relied on comparative effectiveness research to guide their decisions. These agencies often use RWE to answer important post-marketing questions about a drug’s performance in real-world settings. For example, NICE has conducted comparative effectiveness and safety studies using RWE to inform their evaluations.
CMS can learn from the experiences of international HTA agencies in integrating RWE into their decision-making processes. By adopting similar methodologies, CMS can ensure that their price negotiations are informed by the most comprehensive and reliable evidence available.
Challenges in Generating High-Quality RWE
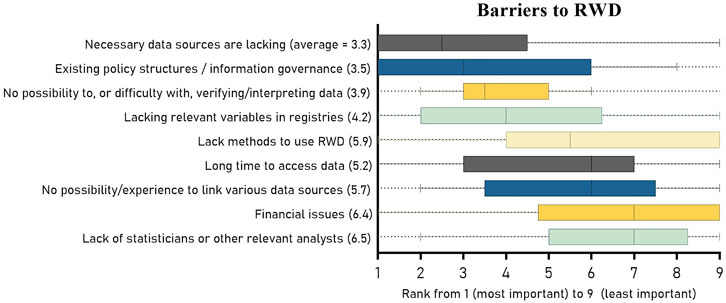
Despite its potential, generating high-quality RWE is not without challenges. One major hurdle is the availability of fit-for-purpose RWD. High-quality data is essential for producing robust RWE studies. CMS must invest in improving its claims data infrastructure to ensure data quality and accessibility.
Moreover, linking CMS claims data with other sources, such as EHRs, can provide a more complete picture of patient outcomes. This linkage is vital for capturing all necessary data elements, including outcomes and confounders. Without a robust RWD infrastructure, the generation of high-quality RWE studies remains challenging.
Recommendations for a Robust RWE Framework
To promote the generation of transparent and high-quality RWE studies, CMS should establish clear standards for evaluating RWE. Agencies such as NICE and the FDA have delineated guidelines defining the criteria for high-quality RWE. These guidelines can serve as a starting point for CMS to develop its own standards.
CMS should also invest in internal experts in fields such as pharmacoepidemiology, health outcomes research, data science, and biostatistics. These experts can help ensure that RWE studies meet the necessary methodological rigour. Also, CMS should develop clear parameters for when it will conduct its own RWE studies and how these studies will comply with established standards.
The Future of RWE in CMS Price Negotiations
The integration of RWE in CMS price negotiations holds great promise. By committing to using RWE and providing clear standards, CMS can incentivise researchers to generate high-quality studies. This, in turn, will ensure that drug pricing and value decisions are based on the best available evidence.
In conclusion, RWE is poised to play a significant role in CMS price negotiations. However, to fully realise its potential, CMS must address the challenges of data quality and accessibility. By investing in a robust RWD infrastructure and establishing clear standards for RWE, CMS can ensure that its price-setting decisions are informed by the most reliable evidence.
