
Unveiling the Potential of Healthcare Innovation Centres
A growing trend in US healthcare systems, innovation centres are creating waves in the sector. These centres, which prioritise the quick creation, operationalisation, and assessment of novel innovations—many of which are digital—are transforming the healthcare industry. They are usually developed in response to issues that healthcare systems encounter, with a shared emphasis on problem-solving and real-time healthcare delivery.
These centres focus on three main areas: rapidly testing, assessing, and scaling domestic initiatives; disseminating creative, effective ideas throughout the healthcare system; and creating new solutions and ideas from data, experiences, and activities. Innovation centres can provide excellent, hyperlocal research and quality improvement when they are operating at peak efficiency.
The Success and Sustainability of Innovation Centres
However, it’s estimated that up to 90% of industry innovation centres fail. Despite this, healthcare innovation centres have seen significant growth in the past decade. The key to their success and sustainability lies in how they define their value and success.
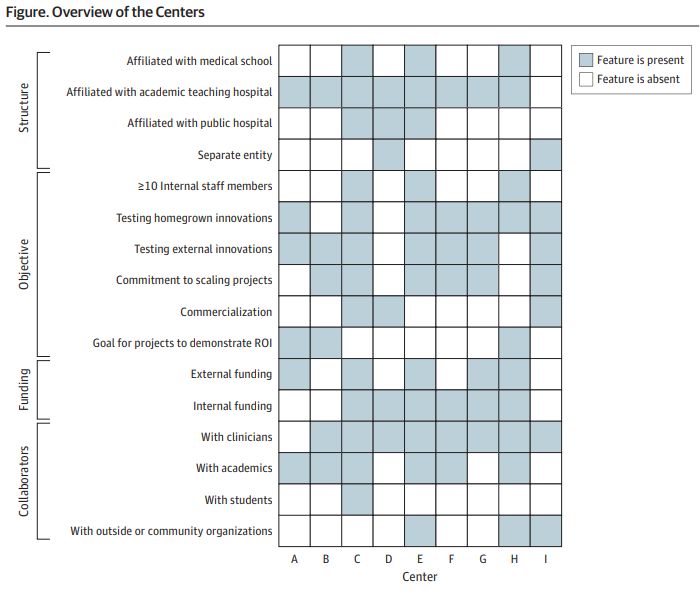
Innovation centres often focus on demonstrating value in a creative way, rather than commercialisation of innovations. They add value to their institutions through their projects and innovations. For instance, they may create a decision support tool in the electronic health record to identify patients who smoke upon hospital admission. This not only benefits the patients but also the institution by meeting quality metrics.
These centres also offer value by “failing fast”, spending less money to understand whether a project or innovation will or will not work. This sets them apart from the wider healthcare organisation, which tends to be slower and more cautious.
Centres for healthcare innovation can become sustainable entities within US healthcare institutions. However, they must work effectively with their institutions to define success more broadly than purely financial return on investment. The field needs to develop more robust means of measuring these nonfinancial benefits for long-term success.
