
Seasonal influenza, a contagious respiratory illness, is a global health concern that can lead to serious complications, especially among vulnerable groups. The World Health Organization estimates that this disease accounts for approximately 290,000 to 650,000 respiratory deaths annually worldwide. In Ireland, the Health Service Executive’s Seasonal Influenza Vaccination Programme offers a free annual influenza vaccine to certain population groups, including adults aged 65 years or older, healthcare workers, those with certain medical conditions, pregnant women, and carers.
Recently, the Health Information and Quality Authority (HIQA) conducted a rapid health technology assessment (HTA) to evaluate whether the vaccination programme should be temporarily expanded for the 2023-2024 season to include individuals aged 50 to 64 years. This follows a similar temporary measure implemented for the 2021-2022 season due to the widespread circulation of SARS-CoV-2.
The rapid HTA, carried out by an Evaluation Team from the HTA Directorate at HIQA, revealed that the vaccine uptake has averaged 28% in those aged 50 to 64 years over the last two influenza seasons. The team found that extending eligibility could result in a modest increase in uptake, provided it is supported by a public health information campaign. The estimated cost of this extension for the 2023-2024 influenza season is approximately €2.3 million, assuming that total uptake in this age group increases from 28% to 35%.
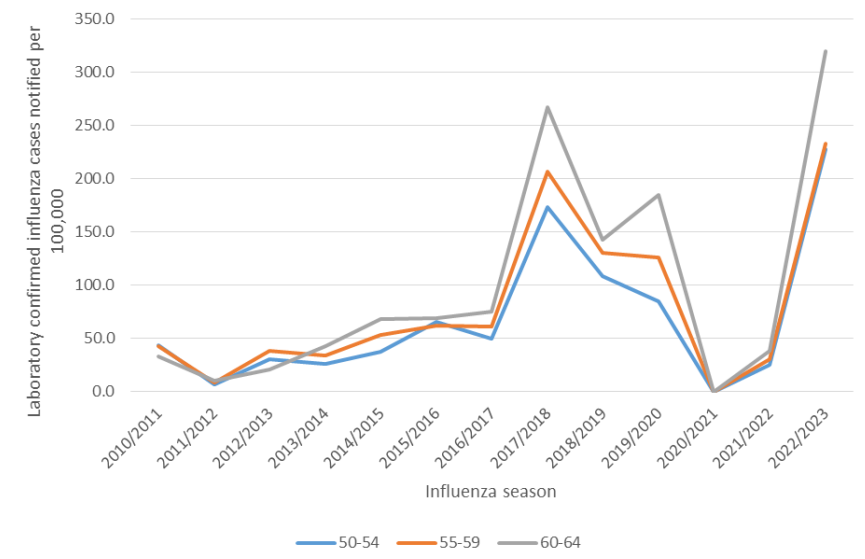
However, the team also noted substantial uncertainty regarding the potential costs and benefits of expanding reimbursement to this age group. Given the relatively modest number of influenza-related hospitalisations in this age group and the substantial year-on-year variability in vaccine effectiveness, the potential for a reduction in demand for hospital care is likely to be small.
Based on these findings, HIQA advises the Minister for Health and the Department of Health to consider limiting the programme extension due to an apparent trend of increasing burden with increased age. If the programme were to be extended just to those aged 60 years and older, the estimated incremental cost for 2023-2024 would be €0.68 million. If extended to those aged 55 years and older, the cost would be €1.43 million.
Should the decision be made to expand the HSE Seasonal Influenza Vaccination Programme, HIQA recommends enhanced data collection and public health information campaigns to empower individuals and support informed decision-making.