
The recent outbreaks of epidemic and pandemic-prone diseases have underscored the importance of timely and effective systems for outbreak detection, notification, and response. Early detection and response are crucial in preventing the escalation of outbreaks of infectious disease. To enhance the identification and control of these threats, ambitious and achievable targets are required.
In response to this need, the World Health Organization (WHO) has developed the Early Action Review (EAR), a performance improvement approach used to evaluate the timeliness of early detection activities and responses to any health event. The EAR, when repeated consistently, establishes the habit of ensuring continual learning and improvement from every event.
EAR is not just an assessment or monitoring tool: it’s a performance management process conducted as early as possible while an event is unfolding. It is used by teams responsible for responding to the event to reinforce the implementation of seven early response actions. These include initiating investigations, conducting epidemiologic analysis, obtaining laboratory confirmation, initiating appropriate case management and infection prevention measures, and establishing a coordination mechanism.
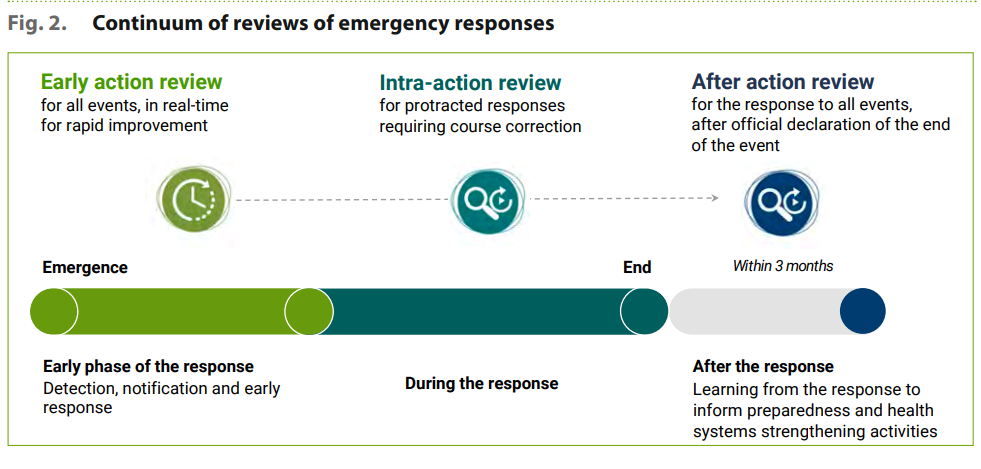
EAR also promotes collaboration and coordination among key stakeholders during the early outbreak response, increasing the likelihood of effective control. When a new outbreak or public health event is identified, stakeholders must coordinate efforts to control the spread of disease and reduce risks or impacts.
The EAR process is designed to find system bottlenecks and fix them quickly. Stakeholders in a response capture data about the timeliness and completion of early response actions as the event unfolds and then identify bottlenecks in the system and enablers of implementation. Documenting these bottlenecks and enablers is critical for prioritising immediate or longer-term remedial actions and identifying best practices.
The WHO has made available five tools to support the conduct of an EAR, which can be downloaded from their website. These tools will be periodically updated, as required. By implementing and improving the EAR process, countries can enhance both their detection speed and response quality, ultimately saving lives and preventing the spread of infectious diseases.