
The English National Institute for Health and Care Excellence (NICE) is a key player in Health Technology Assessment (HTA) worldwide. A report from the Office of Health Economics (OHE) explored NICE’s decision outcomes and their effects on HTA in 12 other countries.
Data showed that NICE decisions are issued relatively quickly, often favouring positive outcomes. However, NICE frequently issued “optimised decisions,” recommendations for using an intervention in a smaller population than licensed but with more favourable cost-effectiveness evidence.
The report also found that NICE’s decisions have a global impact. But, the underlying factors may be more influential than individual technology decisions. NICE’s influence often comes from factors like its reputation as a methods innovator, its decision speed, and the accessibility of its guidance.
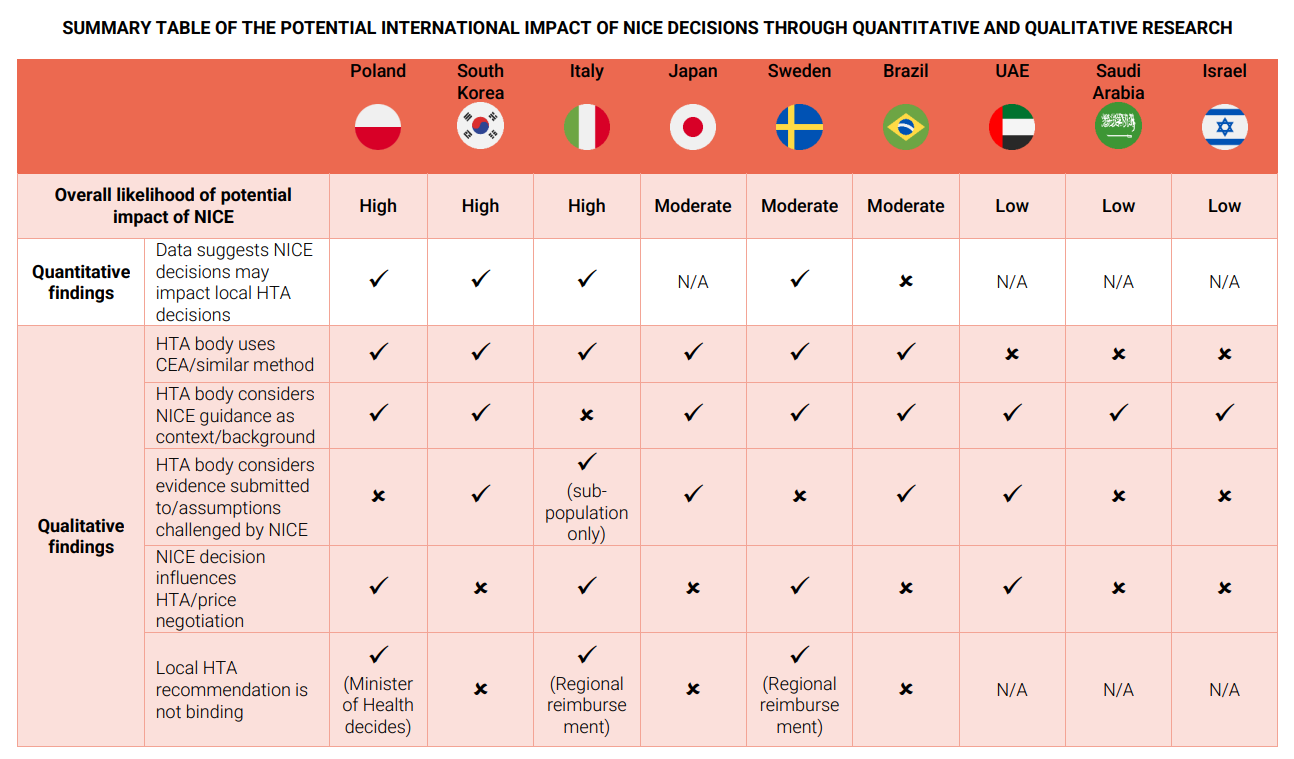
NICE decisions have varying degrees of influence on HTA bodies across different countries:
- In South Korea, NICE’s decisions significantly impact the Health Insurance Review and Assessment Service (HIRA), with external stakeholder groups pressuring HIRA to follow positive NICE recommendations.
- In Brazil, NICE’s decisions also have a strong influence on the National Committee for Health Technology Incorporation (Conitec).
- In Poland, positive NICE decisions do not greatly impact local reimbursement decisions, but negative decisions or issues with the economic model require further validation.
- In Sweden, the Dental and Pharmaceutical Benefits Agency (TLV) considers NICE’s guidance but makes independent decisions.
- In Japan, NICE’s decisions are requested by the Central Social Insurance Medical Council (Chuikyo), but their use and influence are unclear.
- In Italy, the Italian Medicines Agency (AIFA) does not usually consider NICE’s decisions but may refer to technical points raised during NICE’s appraisal for complex cases.
- In Saudi Arabia and the UAE, the Ministry of Health may look at NICE’s decision for context, and in Israel, NICE’s decisions serve as a reference.
While positive NICE decisions are associated with positive outcomes in other countries, negative decisions stand out. Other HTA agencies are less willing to go beyond binary decision outcomes, making NICE’s negative decisions more noticeable.
The future impact of NICE on the global HTA landscape might change due to increased collaborations between HTA agencies and post-Brexit activities.
