
Introduction
Osteoarthritis poses a significant global health challenge, with a growing demand for prosthetic joint replacements due to an ageing population. As the need for joint arthroplasty rises, particularly knee revisions, the economic implications of prosthetic infections are set to escalate. Germany’s projections indicate a substantial surge in geriatric patients and orthopaedic implant utilization. There is an estimated 43% increase in total knee arthroplasty (TKA) procedures by 2050. Additionally, a 90% rise in TKA revision operations is expected.
The Challenge of Prosthetic Infections
Implant failures often originate from factors like instability, mechanical issues, and infections. Notably, periprosthetic infections contribute to increased healthcare costs and prolonged treatment durations. Periprosthetic infections, with rates varying from 0.4% to 2% in primary TKA and 5.6% in revisions, necessitate meticulous management strategies. The prevailing treatment approach involves two-stage revisions. This is a costly and intricate process involving spacer placement and prolonged antibiotic regimens.
Comprehensive Methodological Overview
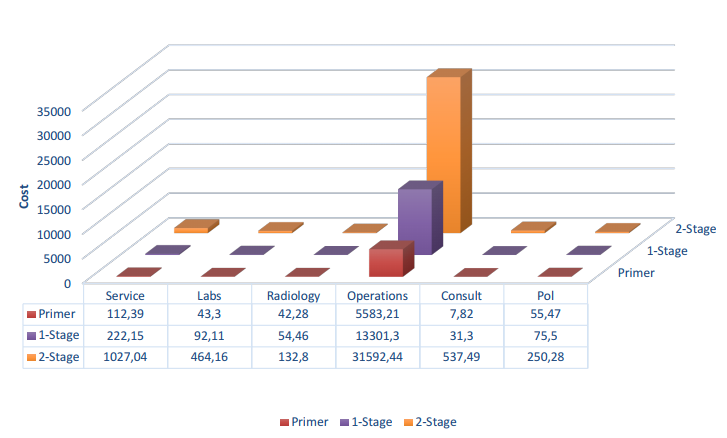
In this study conducted at a specialized tertiary care center in Türkiye from January 1, 2017, to December 31, 2022, the medical records of 524 patients who underwent total knee arthroplasty were reviewed. A total of 76 patients were included in the study and divided into three groups: primary arthroplasty, aseptic loosening revision, and two-stage revision for infections. Post-operative care was standardized for all patients. This included dressing changes, pain management, antibiotic therapy, and rehabilitation. Detailed documentation of operation, anesthesia, consultation, pharmaceutical, laboratory, radiology, and other associated costs was obtained from the hospital’s purchasing and statistics department. Their distribution is given in Figure 1.
Economic Impact and Cost Analysis
The increasing incidence of arthroplasty cases, involving aseptic loosening and periprosthetic infections, indicates a rise in financial burdens for patients, healthcare providers, and institutions. Existing research on cost estimations lacks comprehensive data. There is a need for a closer examination of factors influencing the economic toll of knee revisions. This study explores the detailed cost breakdown of primary arthroplasty, revision surgeries, and the notably expensive two-stage revision procedures for periprosthetic infections.
Discussion and Implications
With advancing technology and demographic shifts, orthopaedic procedures are on the rise. This amplifies the need for cost-effective solutions in managing prosthetic infections and revision surgeries. Notably, the study underscores the imperative of preventing periprosthetic infections to curtail healthcare expenses and enhance patient outcomes. The economic repercussions of periprosthetic infections are substantial. Proactive measures are necessary to mitigate financial strains on healthcare systems and patients.
Addressing the Cost Burden
Efforts to minimize revision surgeries through meticulous patient selection, optimal procedures, and non-operative osteoarthritis management are pivotal. These strategies alleviate the financial strains associated with knee revisions. By adopting a comprehensive approach encompassing preventive strategies and rational treatment selection, the incidence of revisions and associated costs can be minimized.
Conclusion
The economic challenges posed by periprosthetic infections necessitate innovative solutions and research. These will streamline protocols and reduce financial burdens. As the demand for knee arthroplasty rises, healthcare systems must prioritize cost-effective measures. This is essential to ensure the sustainability of public financial resources, particularly in public healthcare institutions.
