The Silent Burden of Early-Stage CKD
Chronic kidney disease (CKD) is a silent epidemic, affecting 9.1% of the global population. Both end-stage renal disease (ESKD) and cardiovascular disease (CVD) are significantly more likely to occur as a result of this risk factor. CKD often progresses into ESKD or triggers CVD, leading to substantial economic burden in healthcare expenditures. Furthermore, many individuals with early-stage CKD remain undiagnosed, potentially escalating medical care costs. Despite its significance, we lack a clear understanding of the economic cost associated with early-stage CKD.
Economic burden of early-stage CKD
Researchers have found that advanced-stage chronic kidney disease, which is often diagnosed by medical professionals, is associated with increased utilisation of healthcare services. On the other hand, due to the constraints of the data gathering process, these studies frequently underestimate the economic burden of early-stage chronic kidney disease. A statewide annual health checkup program in Japan has made it possible to investigate the early stages of CKD in the general population. This is a one-of-a-kind opportunity. It is possible for physicians, patients, policymakers, and public health sectors to prioritise interventions for this population if they have an awareness of the economic impact of chronic kidney disease in its early stages.
Excess Health Care Utilisation in Early-Stage CKD
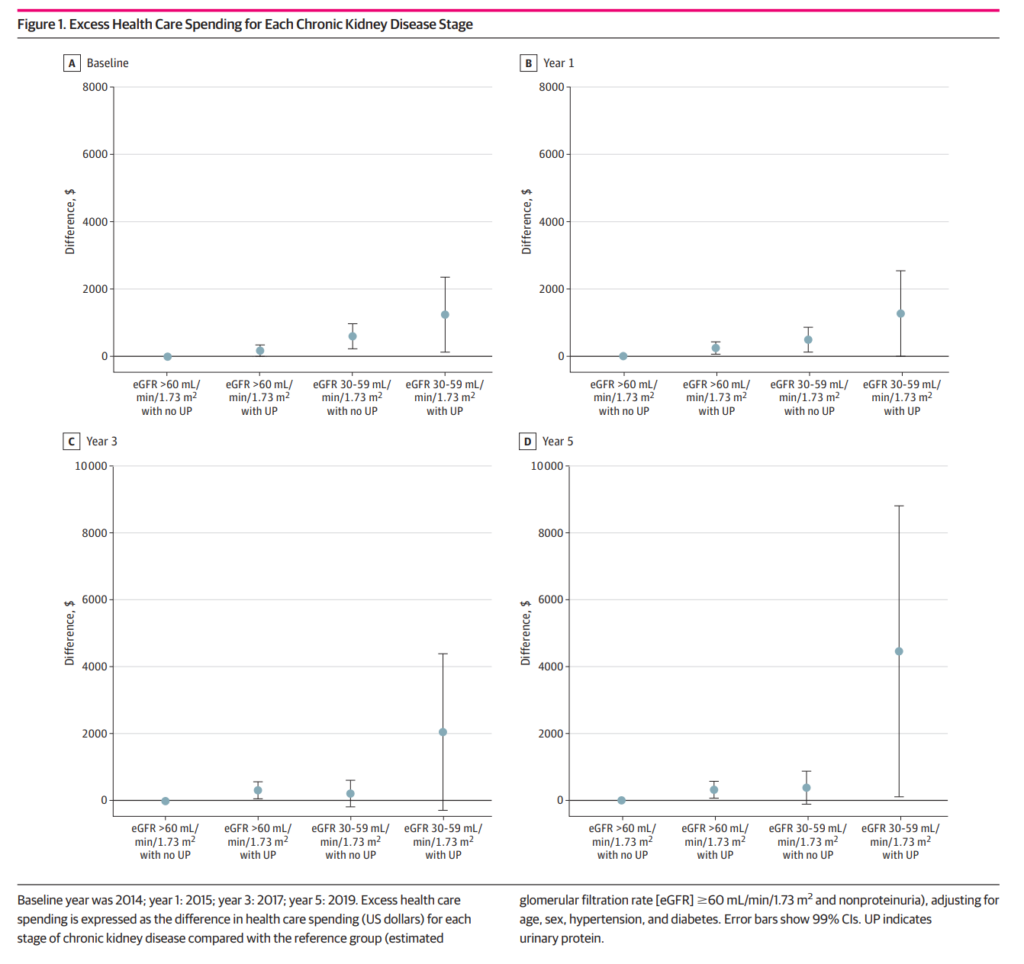
Kyoto University’s Graduate School of Medicine recently conducted a study on the overuse of healthcare services. They focused on healthcare spending, outpatient treatment days, and hospitalisations among people in the early stages of CKD. The screening results showed that 5.3% of the participants had early-stage CKD. Over a five-year period, the study observed excessive healthcare use due to proteinuria and a slightly reduced eGFR. The costs ranged from $6-$350 for proteinuria, $233-$983 for a reduced eGFR, and $134-$2373 for a combination of both. These results emphasise the importance of preventative public health and clinical measures. These measures can slow CKD progression and lower the economic burden of early-stage CKD.
The Need for Effective Interventions
The study found a consistent link between early-stage CKD and increased healthcare costs over five years. It’s clear we need effective medical interventions to reduce this high healthcare use. However, the complexity of CKD-related activities calls for in-depth research to find beneficial interventions. Since CKD significantly increases the risk of CVD, preventing CVD could help lower healthcare use in CKD patients.
The Way Forward
This study marks the first investigation into the high healthcare spending linked to early-stage CKD in the general population. The findings highlight the need for preventative measures to curb the onset and progression of CKD. This is crucial to reduce the CKD-associated healthcare burden and ensure the sustainability of the healthcare system.