
The COVID-19 pandemic has had far-reaching implications, with recent reports indicating a surge in the incidence of pediatric diabetes. To gain a clearer understanding of this association, a systematic review and meta-analysis of various studies published in English was conducted.
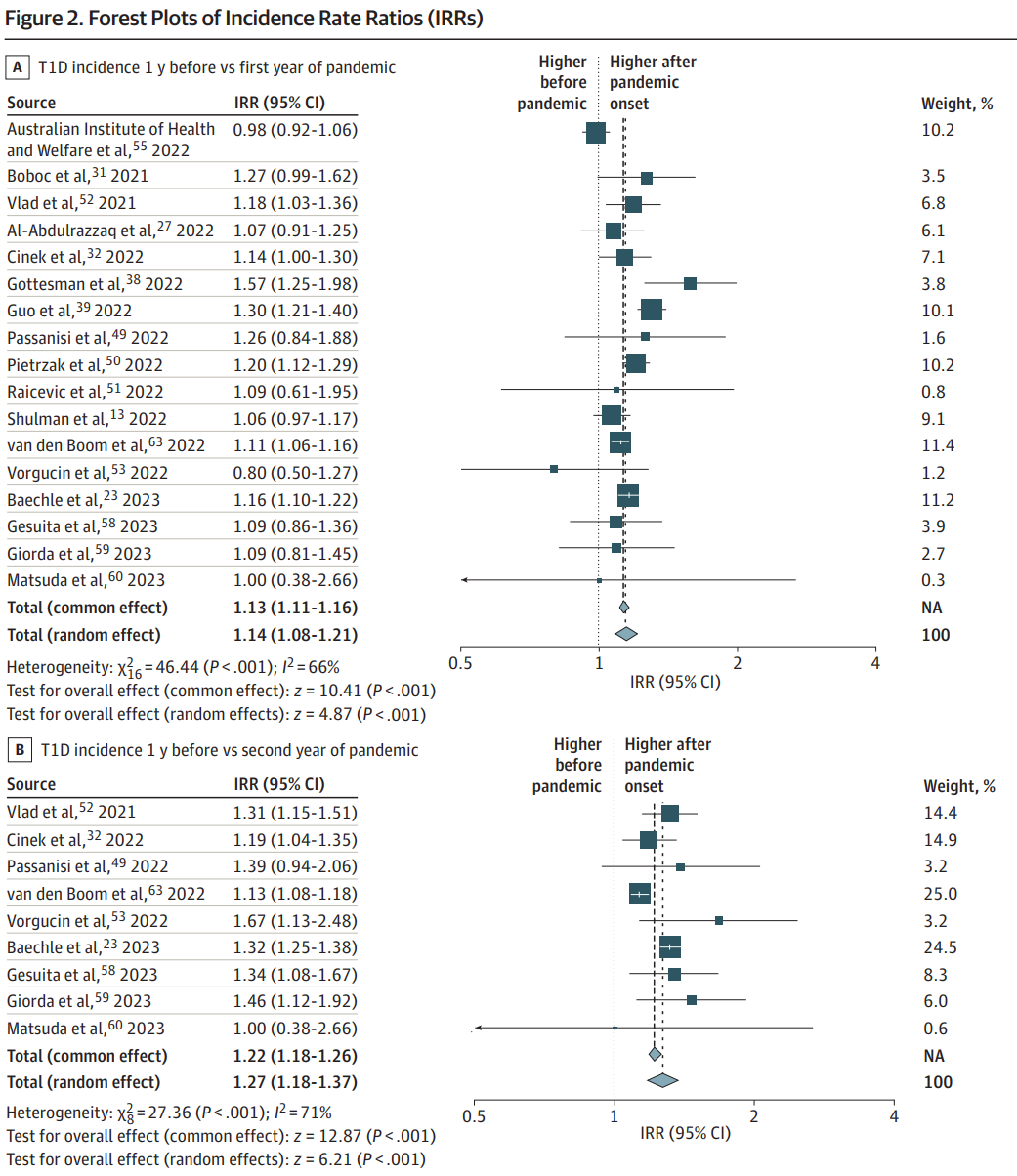
The analysis included 42 studies, covering 102,984 incident diabetes cases. The results revealed a significant increase in the incidence rate of type 1 diabetes and diabetic ketoacidosis (DKA) in children and adolescents during the first two years of the pandemic compared to the pre-pandemic period.
While the exact mechanism linking COVID-19 to the onset of diabetes remains unclear, potential factors might include lifestyle changes, stress, social isolation, and disruptions in healthcare access due to pandemic containment measures.
These findings underscore the urgent need for increased resources and support for the growing number of children and adolescents with diabetes. Future studies are required to determine whether this trend continues, and to shed light on the possible underlying mechanisms explaining these temporal changes.
The increased incidence of DKA at diagnosis highlights the need to identify gaps in the pathway from the onset of diabetes symptoms to diagnosis. This information is crucial for developing effective strategies to prevent DKA at diagnosis in children.