In a groundbreaking study published in Journal of Pharmaceutical Policy and Practice volume, an analysis was conducted on the R&D costs of oncology medicines. The analysis, based on data from company filings from 1997 to 2020, indicates that a precision approach to oncology medicine development using Companion Diagnostic (CDx) could significantly reduce costs and increase ROI.
Despite some missing data, primarily from larger pharmaceutical companies that have acquired smaller firms, the study still provides a robust picture of the R&D landscape. They found that the total R&D spend to develop an oncology medicine (inconsistent use of CDx) is $4432.1 m, a figure that is significantly reduced when a CDx-guided precision oncology approach is adopted.
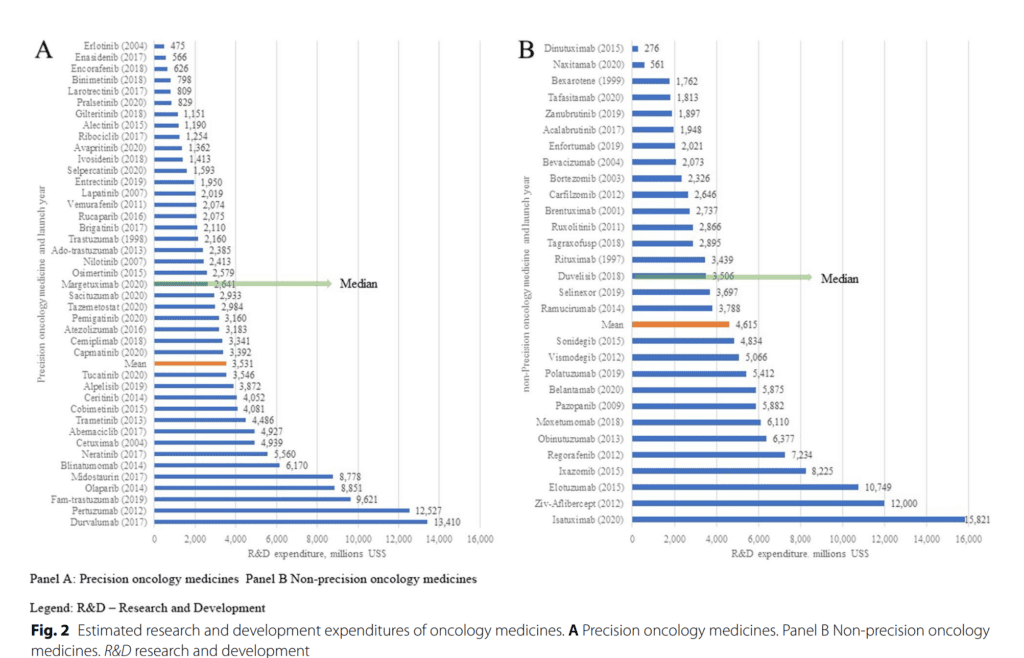
While the World Health Organization (WHO) has called for more transparency in R&D costs, this study highlights the need for standardisation in reporting R&D spend. This is crucial for health systems to make well-informed decisions on healthcare policy and for the pharmaceutical industry to recover R&D costs and continue innovating.
Findings suggest that precision oncology medicines guided by CDx are not only less expensive to develop but also offer better value to stakeholders, including patients, payers, and the pharmaceutical industry. This approach not only reduces R&D spend but also accelerates the reimbursement process and requires a rethinking of market access strategies.
The implications are far-reaching, impacting drug discovery, market access, health outcomes, and industry policy. As the pharmaceutical industry realises the cost-effectiveness of precision oncology medicines, it presents challenges to ensure equitable treatment provision for all patients.
This study emphasises the need for a flexible CDx development framework to truly benefit patients and ensure that R&D spend in oncology medicine development is affordable to health systems. The industry should revisit the commercial model for precision medicines and their associated diagnostic/prognostic tests to capitalise on the benefits of precision medicine.
Reference url