
Introduction
Respiratory Syncytial Virus (RSV) is a contagious respiratory illness prevalent in fall and winter, especially impacting infants, young children, and vulnerable individuals. In the Kingdom of Saudi Arabia (KSA), RSV significantly burdens public health, particularly affecting children under 5. Globally, there were 33.1 million RSV cases, 3.2 million hospitalisations, and 59,600 deaths, with children under 6 months accounting for 45% of severe outcomes in 2015. In the KSA, RSV peaks in winter, causing 23% of paediatric respiratory illnesses from 2001 to 2015. Globally, almost €5 billion is spent on direct medical expenses linked to RSV-induced lower respiratory tract disease.
Treatment Landscape
The KSA currently has no RSV prevention treatments for otherwise healthy children. Palivizumab is the only prophylaxis product and is limited to high-risk patients with specific conditions. The approval of nirsevimab, a monoclonal antibody with an extended half-life and rapid protection onset, offers a novel option. The Food and Drug Administration (FDA) has approved nirsevimab for newborns and infants in their first RSV season and extended clearance up to 24 months for high-risk children. The Centers for Disease Control and Prevention (CDC) recommends nirsevimab for all infants per the FDA label, but the Saudi Food and Drug Authority has not yet approved it. This study evaluates the potential public health and economic benefits of implementing a universal nirsevimab immunoprophylaxis strategy compared to the current standard of care in the KSA.
The HARMONIE Study: Nirsevimab’s effectiveness
The HARMONIE study, a phase 3b European clinical trial, showed nirsevimab reduced inpatient hospitalisations by 83.2% in infants. Researchers used this reduction to define the percent decrease in RSV acute medically attended lower respiratory tract disease (MA-LRTDs). Outpatient efficacy data came from randomised, placebo-controlled trials, including a phase 2b study in preterm infants and the phase 3 MELODY study in term infants. The phase 2b study highlighted nirsevimab’s effectiveness in high-risk preterm infants, while the MELODY study validated its efficacy in term infants. Researchers integrated these results to establish a robust efficacy profile for nirsevimab, demonstrating its significant impact in reducing RSV MA-LRTDs in both inpatient and outpatient settings. Consequently, nirsevimab shows potential public health benefits across different infant subpopulations.
Cost Effectiveness Analysis: Nirsevimab Immunoprophylaxix vs Standard of Practice (SoP)
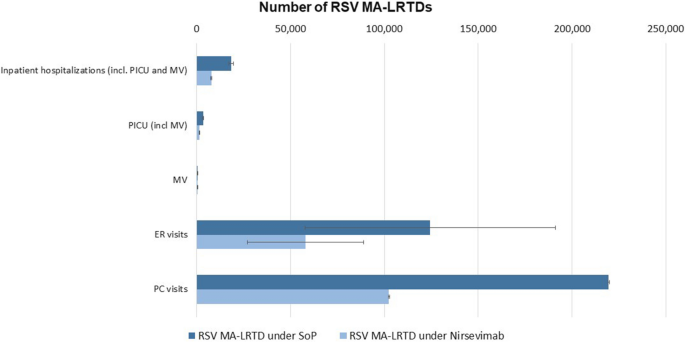
A decision-analytic model was used to assess the burden of RSV-induced health events under the current SoP and with nirsevimab immunoprophylaxis. The model assessed healthcare resource utilisation, including inpatient hospitalisations, paediatric intensive care unit (PICU) admissions, mechanical ventilation (MV) requirements, emergency room (ER) visits, and primary care (PC) visits, along with associated costs under the SoP. Lastly, the model then evaluated the number of cases averted and costs saved with universal nirsevimab compared to the SoP.
Key Findings
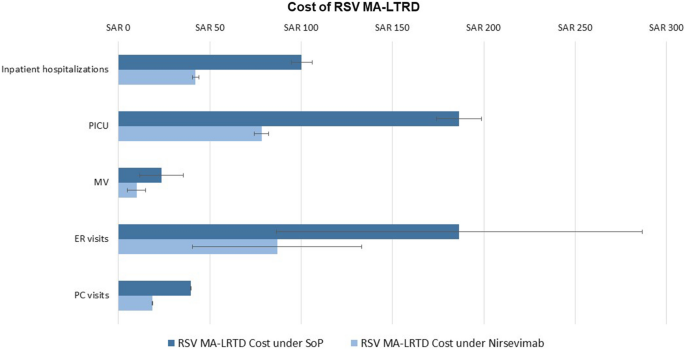
Nirsevimab is cost-effective in managing MA-LRTDs caused by RSV due to its substantial reduction in both health events and associated costs. The model evaluated the impact of nirsevimab and found that it reduces inpatient hospitalisations, PICU admissions, mechanical ventilation, emergency room visits, primary care visits, RSV-related mortality, and recurrent wheezing by 53% to 59%, significantly alleviating the clinical burden. Financially, it reduces total hospitalisation costs from SAR 280.5 million–340.2 million to SAR 119.7 million–141.0 million and total MA-LRTD costs from SAR 466.1 million–606.7 million to SAR 200.2 million–271.2 million. These reductions demonstrate that nirsevimab not only improves health outcomes but also offers substantial economic savings, making it a cost-effective strategy for RSV management in infants.
Conclusion
Future research should focus on evaluating the incremental cost-effectiveness ratios (ICERs) and quality-adjusted life years (QALYs) associated with nirsevimab. These metrics will help quantify the economic and health benefits of implementing a universal nirsevimab immunoprophylaxis strategy in the KSA. By assessing ICERs and QALYs, researchers can provide a comprehensive understanding of the long-term value of nirsevimab, including its impact on healthcare costs and quality of life for infants and families. Policymakers should consider these findings to make informed decisions about integrating nirsevimab into national immunisation schedules, ultimately improving public health outcomes.
