
Introduction
Real-World Evidence (RWE) is increasingly recognised for its potential to complement traditional randomised controlled trials (RCTs) in regulatory decision-making. Initially used for post-approval safety studies, RWE now supports new drug applications (NDA), particularly for orphan drugs or high unmet needs. In the approval of abaloparatide, marketed as Eladynos, for osteoporosis treatment in the European Union (EU), RWE played a crucial role. The initial marketing authorisation application (MAA) faced challenges, including concerns about fracture efficacy and cardiovascular safety. By utilising additional interventional and observational studies, along with post-marketing data, the resubmitted application addressed these issues, leading to the drug’s approval. This case study underscores the evolving acceptance of RWE in regulatory processes and its vital role in filling clinical data gaps.
Real-World Data in EU Medicines Regulation
The European Medicines Agency (EMA) embraces RWE, emphasising patient safety and fostering innovation. Strategic roadmaps demonstrate the shift towards a robust pharmacovigilance system and the incorporation of RWE in benefit–risk evaluations. The EMA and Heads of Medicines Agencies (HMA) have issued strategic 5-year roadmaps since 2005, focusing on strengthening the protection of human health and facilitating innovation.
Case Study: Abaloparatide’s Approval Journey
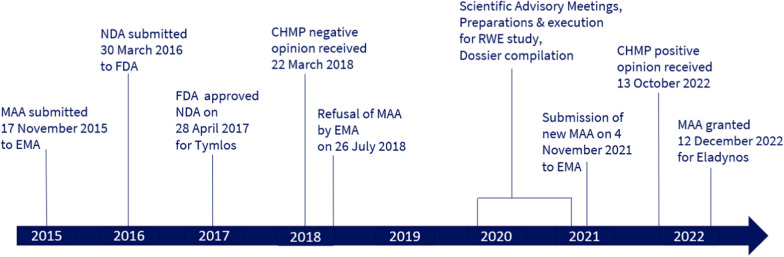
Abaloparatide, marketed as Eladynos, encountered regulatory hurdles due to efficacy and safety apprehensions. The initial MAA submitted in 2015 faced rejection due to concerns about fracture efficacy and cardiovascular safety. The pivotal Phase 3 study (ACTIVE) failed to demonstrate a statistically significant effect on non-vertebral fractures (NVF) versus placebo, and there were concerns about potential safety risks associated with transient heart rate increases.
The ACTIVE study, a randomised, double-blind, placebo-controlled Phase 3 trial, aimed to establish the efficacy and safety of abaloparatide in postmenopausal women with osteoporosis. While the study showed a significant reduction in vertebral fractures, it did not achieve statistical significance for nonvertebral fractures (NVF) due to the exclusion of data from two clinical sites over Good Clinical Practice (GCP) concerns. This exclusion reduced the study population by 16%, impacting the overall results. Moreover, there were concerns about cardiovascular safety, particularly the transient increase in heart rate observed in some patients.
By integrating RWE, additional interventional and observational studies, and post-marketing data, the resubmitted application showcased efficacy in reducing fractures and comparable cardiovascular safety to teriparatide. New data included a dual-energy X-ray absorptiometry (DXA)-3D-based modeling study, a histomorphometry study, a Japanese Phase 3 bridging study, and a retrospective observational cohort study using data from the Health Claims database. These studies provided additional evidence on the safety and efficacy of abaloparatide to reduce fracture risk at both vertebral and nonvertebral sites.
Regulatory Approval Process
The European Commission granted marketing authorisation for Eladynos, acknowledging the comprehensive data presented. The inclusion of a Post-Authorisation Safety Study (PASS) further addressed remaining concerns, ensuring continuous safety monitoring. The Committee for Medicinal Products for Human Use (CHMP) reviewers considered the additional data, including post-marketing data from the US, supportive of efficacy and safety. The observational study demonstrated comparable effectiveness between abaloparatide and teriparatide, including more than 22,000 patients with almost 650 NVF.
Conclusion
The integration of RWE into the regulatory framework for medicinal products marks a significant advancement in drug development and approval. This case study exemplifies the evolving acceptance of RWE in regulatory decisions, highlighting its pivotal role in supporting the approval of new products like abaloparatide for osteoporosis. The collaboration between industry and regulators underscores RWE’s potential to address gaps in traditional clinical trials, especially in areas with high unmet medical needs. The EMA’s forward-thinking approach in incorporating RWE into benefit-risk evaluations enhances the robustness and relevance of clinical evidence. As the regulatory landscape evolves, it is crucial for pharmaceutical companies, healthcare providers, and regulatory bodies to collaborate and leverage RWE alongside traditional RCTs. This holistic approach will expedite the availability of innovative therapies, ensuring their safety and efficacy in real-world settings, ultimately improving patient outcomes and advancing public health.
