Introduction
In healthcare, Precision Medicine (PM) represents innovation, tailoring medical treatments based on individual genetic profiles and leveraging data and genomics. This advancement has evolved diagnostic and prognostic capabilities, enabling early disease detection through biomarkers and personalised therapeutic interventions. By establishing a competency framework for PM, we are able to build a more robust network which allows for better outcomes and reducing costs.
Challenges in Integration
The workforce in the Medical Technology and Pharmaceutical (MTP) industry plays a crucial role in translating new technologies into clinical applications, requiring expertise across domains from clinical research to regulatory affairs. However, integrating PM competencies into this workforce presents challenges due to diverse educational backgrounds and varying skill requirements across industry roles. Therefore, the introduction of the PM competency framework for the MTP industry serves as a strategic initiative to enhance skills and drive innovation in precision medicine practices.
Importance of Competencies
Competencies are vital for effective job performance, defining job-specific requirements and ensuring task completion. Establishing a competency framework is essential to outline the skills and attitudes necessary for professional practice, providing a foundation for tailored learning and skill development.
Proposed PM Competency Framework for the MTP Industry
While competency frameworks exist for healthcare providers and related fields, there is a gap in defining competencies for MTP industry professionals. This paper introduces a PM competency framework tailored for the MTP industry to enhance technical skills and cross-functional capabilities, enabling the seamless translation of PM discoveries into clinical settings.
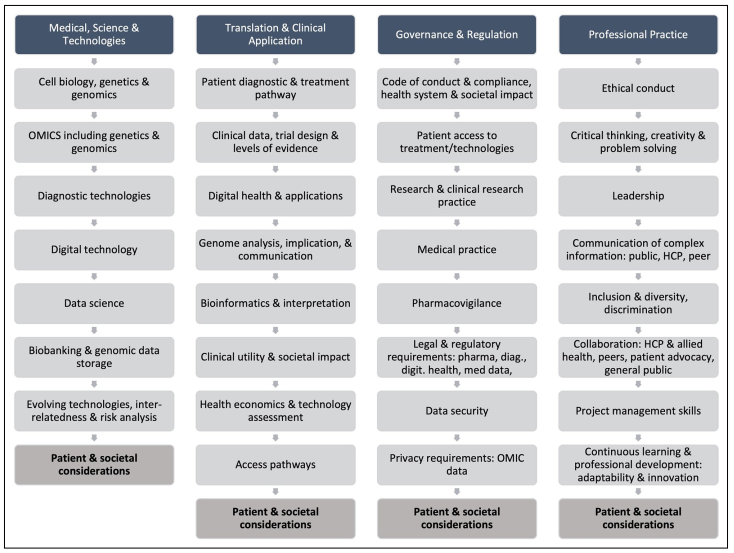
Cultivating a Skilled Workforce
The framework aims to cultivate a workforce skilled at integrating genomic information into daily practices, fostering a culture of continuous learning and innovation across the MTP industry. Key components of translational PM in the pharmaceutical sector include multi-omics profiling, AI integration, and patient-centric companion diagnostics, requiring ongoing education and upskilling of the workforce.
Addressing Industry Gaps
Existing competency frameworks in the MTP industry often lack references to PM, innovation, and continuous learning. The proposed framework includes ethical principles, industry-specific considerations, and emerging competencies in digital health and data science, aligning with industry demands and the evolving healthcare sector.
Leadership and Implementation
Leadership in PM is crucial for driving industry progress, necessitating individuals with complex reasoning, adaptive thinking, and effective communication skills. The competency framework, derived from research and industry feedback, covers domains such as medical science, translation, governance, and professional practice, catering to the diverse needs of the MTP industry.
Advancing Precision Medication Practices with HEOR Integration
The adoption of PM is anticipated to have a profound impact on healthcare costs. A key component of the competency framework must therefore include the ability to evaluate the economic implications of PM interventions. Industry professionals need to be proficient in assessing how personalised treatments can lead to cost savings by reducing trial-and-error prescribing, lowering rates of adverse drug reactions, and providing more effective disease management. Equipped with health economic skills, professionals can contribute to a more cost-efficient healthcare system that maximises patient outcomes while managing expenditure.
Conclusion
The PM competency framework, with its emphasis on economic implications, equips the MTP industry with essential skills to ensure that precision medicine advances are not only at the forefront of scientific innovation but also cost-effective for healthcare systems. By incorporating the principles of Health Economics and Outcomes research (HEOR), professionals can help to navigate the complex interplay between cutting-edge treatments and the economic realities of healthcare delivery, ultimately contributing to a more sustainable and affordable future for personalised patient care.
