
The Current Opioid Crisis and the Treatment of Post-Caesarean Pain
Systemic opioids are the medication that is utilised the most frequently, despite the fact that pain management following a caesarean delivery is a complicated issue. Having said that, this method is not without its flaws. The overprescription of opioids is a key cause, and it is estimated that 1.2 million of the caesarean births performed annually in the United States result in the disposal of 12 million unneeded tablets. There is a high likelihood that these unused tablets will be abused, which will contribute to the continuing opioid problem.
A Novel Technique for the Management of Pain
A recent study found that devices that use high-frequency electrical stimulation could be an effective solution to the problem. According to the findings of the study, women who had caesarean deliveries followed by transcutaneous treatment using a high-frequency electrical stimulation device required around 47 percent less opioid analgesic medication while they were in the hospital. In addition, these individuals reported levels of discomfort that were comparable to those reported by patients who had used a sham device.
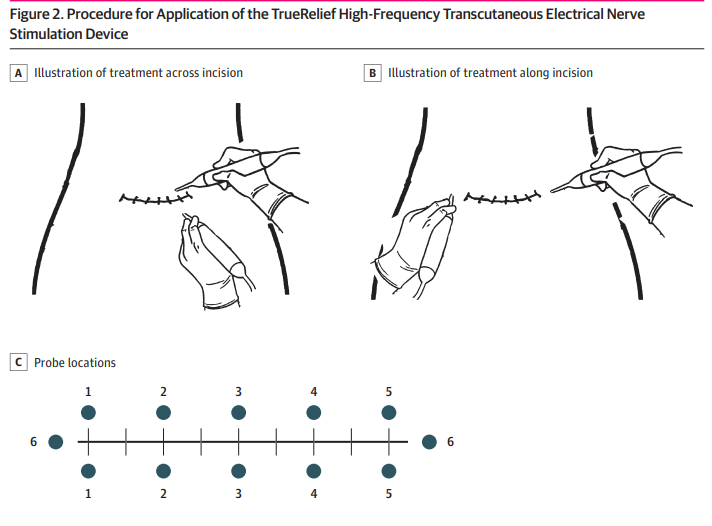
High-frequency electrical stimulation devices
This breakthrough has the potential to constitute a key step towards lowering the use of opioids, avoiding overprescribing, and preventing dependence from developing. Because it is estimated that 36,000 to 72,000 people in the United States develop a dependence on opioids each year after having a caesarean delivery, finding effective measures to reduce opioid use continues to be a public health priority.
