The Rising Costs and Patent Exclusivities of GLP-1 Receptor Agonists
GLP-1 receptor agonists are a crucial class of medications used in the treatment of type 2 diabetes and obesity. Despite their importance, these drugs have seen a significant price increase, with mean monthly net prices rising from approximately $200 in 2007 to over $600 in 2017. Manufacturers have earned more than $10 billion on GLP-1 agonists in the US alone in 2021.
The lack of generic competition for these drugs is primarily due to manufacturers obtaining patents and nonpatent statutory exclusivities. These government-granted rights, which typically last 20 years from the date of filing, allow manufacturers to exclude potential competitors from selling versions of the protected product. This results in high prices for extended periods and poses a significant barrier to the entry of generic drugs into the market.
The Need for Regulatory Changes to Facilitate Generic Competition
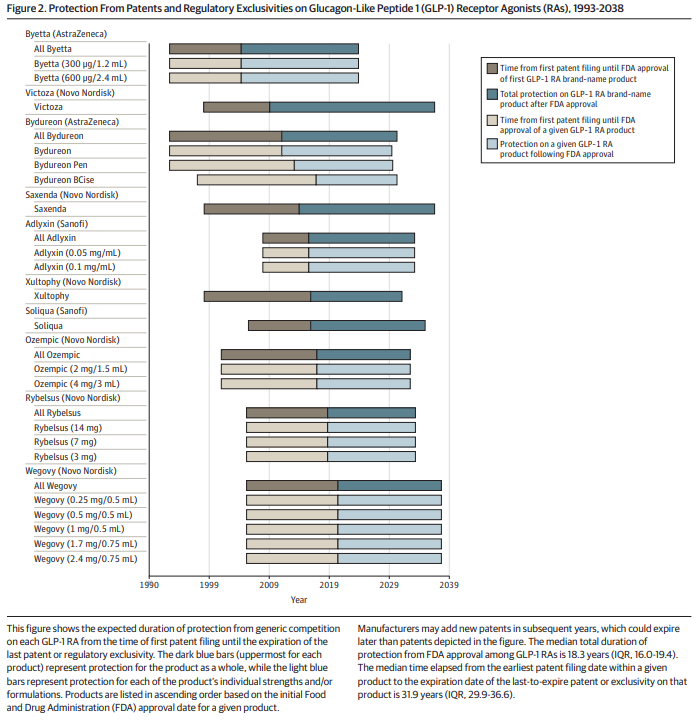
The current patent and regulatory system for drug-device combinations, such as GLP-1 receptor agonists, may be delaying generic competition. It is crucial to address the patent and exclusivity landscape for these drug-device combinations to facilitate generic competition and lower costs.
Lawmakers and regulators need to develop solutions that promote the timely entry of generic drug-device combinations. This would allow manufacturers to earn reasonable returns for limited periods while ensuring more patients can benefit from lower costs and improved access to these useful drugs.
The rapidly expanding market for GLP-1 receptor agonists, coupled with the high costs and patent exclusivities, underscores the need for regulatory changes to ensure these life-saving drugs are affordable and accessible to all.
