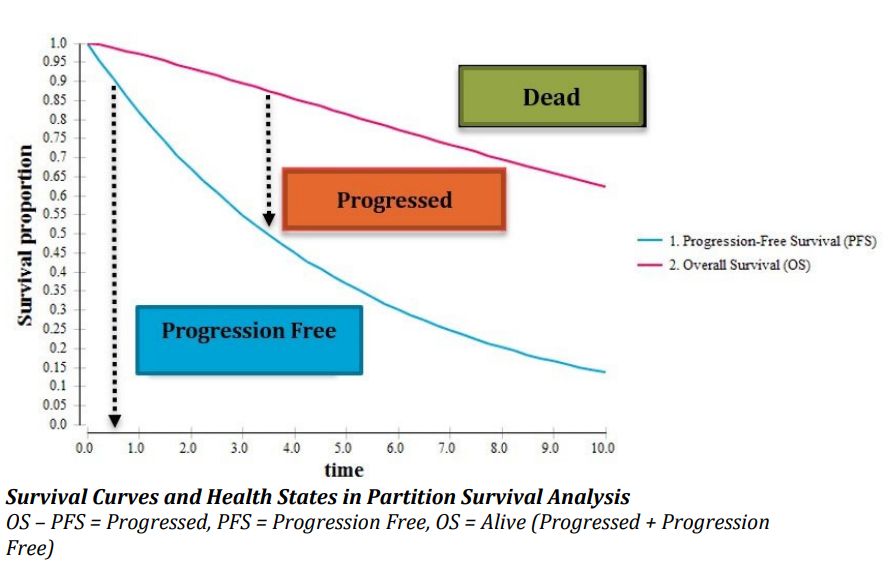
A health technology assessment (HTA) of treatment regimens for patients with multiple myeloma in Norway was done. The goals of treatment are to prolong life, achieve a strong response without side effects, and maintain quality of life.
The article discusses the uncertainty surrounding the results of a health economic analysis of treatment regimens for multiple myeloma. The uncertainty is due to reliance on component network meta-analysis and lack of access to patient level data. The methods used to derive underlying survival curves may overestimate treatment costs due to inaccurate accounting for dose reductions during treatment.
The difficulty in drawing clear conclusions about the most effective treatment regimen for patients with refractory or relapsed multiple myeloma is clear. The six triplet combinations mentioned have favourable hazard ratios for overall survival, but there is substantial uncertainty in the evidence. Cost-effectiveness results are also uncertain and need to be viewed in the context of each reference group. Only seven treatments were not dominated by others. Patients with RRMM may lose 12-15 healthy life-years, and there is a high degree of uncertainty in the cost-effectiveness results.