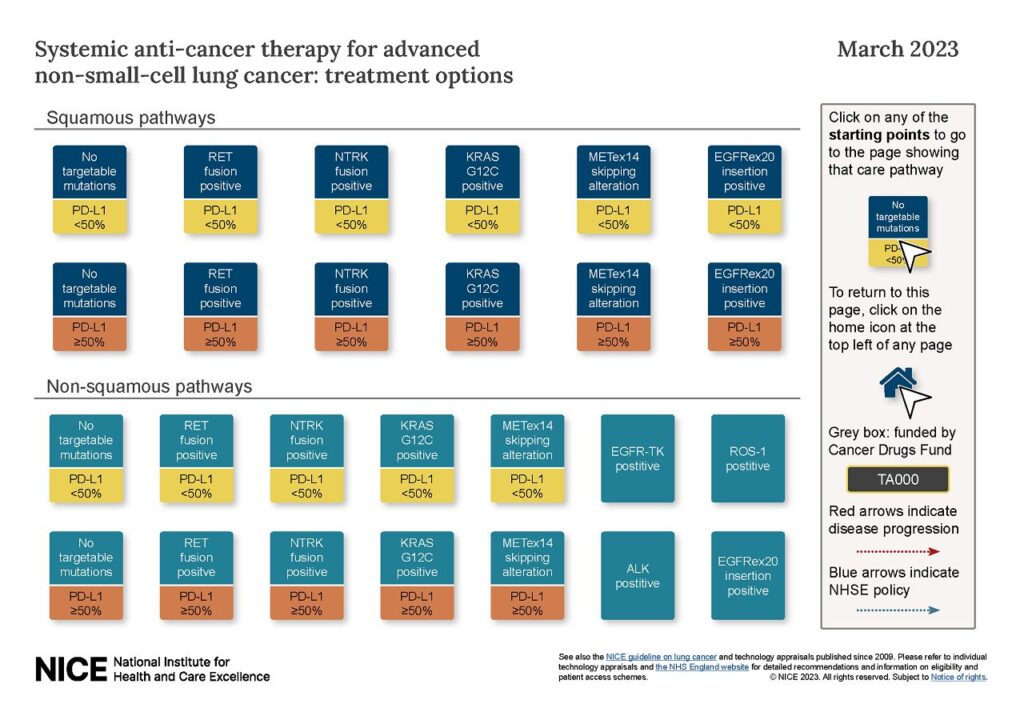
NICE guidelines have added the guidance on mobocertinib to the systemic anti-cancer therapy treatment pathways for advanced non-small-cell lung cancer.
They would like your feedback on how the treatment pathways present content from different sources (NICE technology appraisal guidance, guideline recommendations, and expert input). To comment, go to the link below on systemic anti-cancer therapy for advanced non-small-cell lung cancer and fill in the pop-up survey.
Recent Posts
Aspen Pharmacare Financial Growth: Navigating H1 2026’s Transitional Financial Landscape
Aspen Pharmacare Financial Growth Powers Through Strategic Shifts
Aspen Pharmacare's financial growth shines in its H1 2026 results, driven by resilient Commercial Pharmaceuticals,
South Africa’s Push for Local Lenacapavir Production to Enhance HIV Prevention
South Africa has launched a bold bid to enable local lenacapavir production, targeting the twice-yearly injectable long-acting HIV prevention drug from ...
Urgent Call for Enhanced Precision Oncology Access in Europe
EFPIA White Paper: Unlocking Precision Oncology Access in Europe
The EFPIA Oncology Platform's white paper, "Advancing Precision Oncology Treatment and Testing Across Europe," launched on March 5, 2026, at a high-level...