
Liquid biopsy colorectal cancer screening is gaining traction in Abu Dhabi. The Department of Health plans to implement a new liquid biopsy Shield Test through its Comprehensive Screening Program (IFHAS). The DoH will introduce this innovative approach immediately for eligible UAE nationals, complementing existing methods like colonoscopy and FIT.
Transforming Screening Approaches
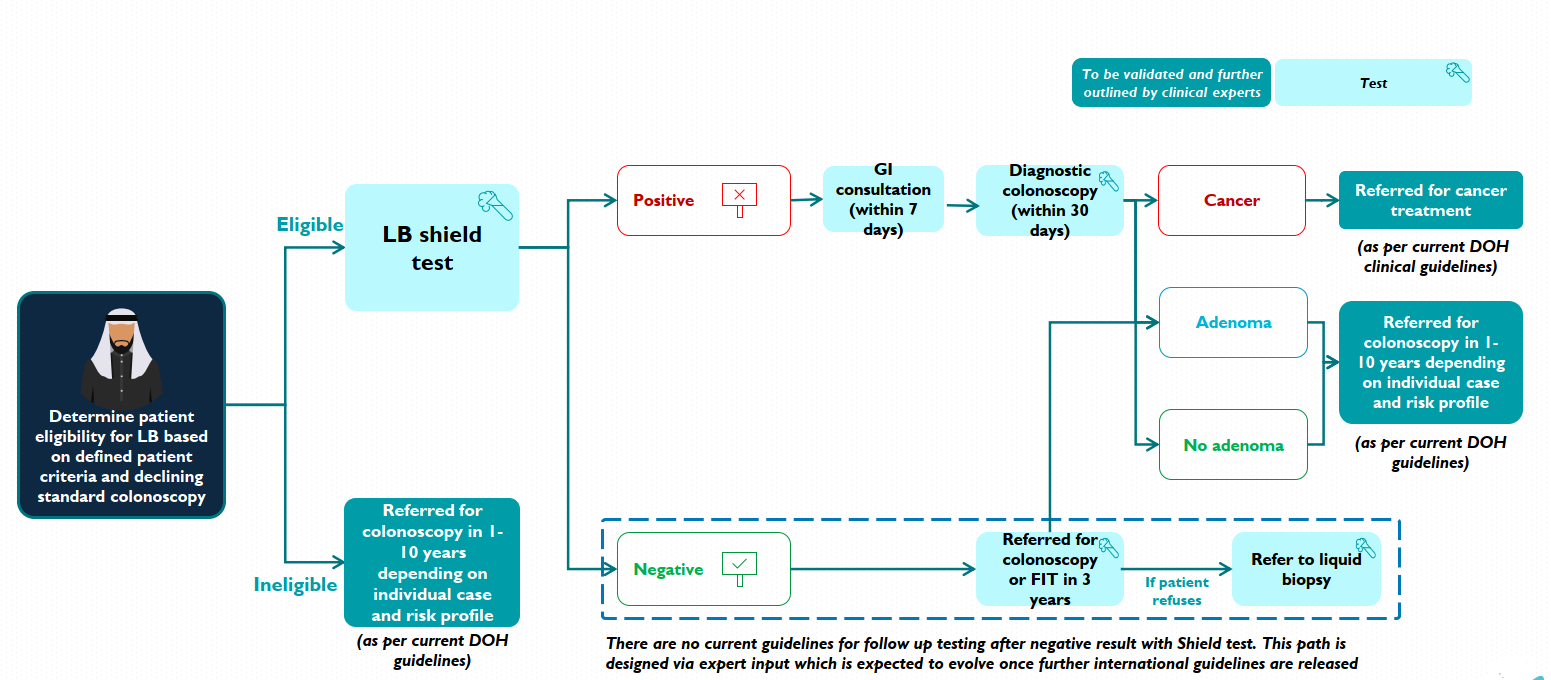
The integration of the liquid biopsy test into Abu Dhabi’s IFHAS program marks a pivotal shift in colorectal cancer detection strategies. As a new screening tool, it aims to enhance early detection through a multi-modal approach, not replacing colonoscopy and FIT. The IFHAS program restricts eligibility to individuals aged 40 and above. High-risk populations, such as those with a personal history of colorectal cancer, genetic disorders, or inflammatory bowel diseases, are excluded.
A Step Beyond Traditional Methods
The introduction of liquid biopsy screening in Abu Dhabi represents a notable advancement beyond conventional methods. These include fecal immunochemical testing or colonoscopy, as recommended by the World Health Organization for adults aged 50-70. However, the US Preventive Services Task Force currently advises against blood-based biomarker tests due to insufficient evidence.
Implications for Healthcare Economics and Market Access
Liquid biopsy could significantly impact health economics by offering a less invasive initial screening option. This may lower the overall costs of colorectal cancer screening programs. A thorough evaluation of its cost-effectiveness compared to traditional methods is still needed. Additionally, this initiative may accelerate the adoption of liquid biopsy technologies in other healthcare systems, expanding the market for such diagnostic tools.
Influencing Global Health Insurance Practices
The inclusion of liquid biopsy in Abu Dhabi’s national screening program could set a new precedent for reimbursement policies globally. It may influence global health insurance practices. Effective implementation could provide valuable insights for other healthcare systems aiming to improve early detection of colorectal cancer.
As the screening method rolls out in Abu Dhabi, continuous monitoring and evaluation of its outcomes will be essential. This will determine its long-term effectiveness and impact on population health, ensuring the promise of liquid biopsy colorectal cancer screening is fully realized.
