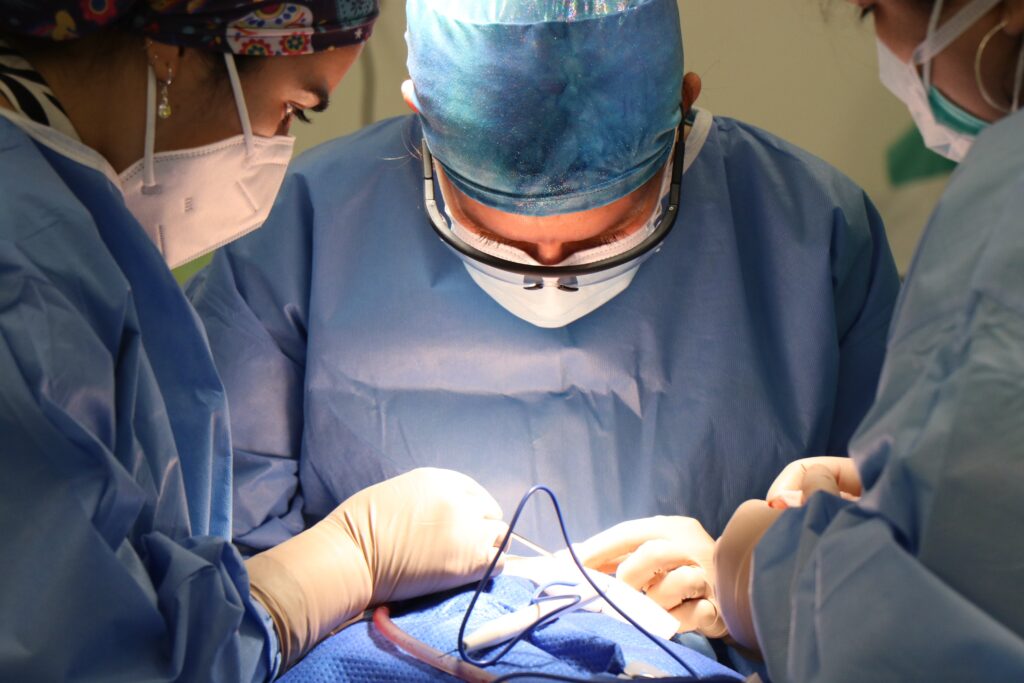
🚀 Are we on the brink of a revolution in DLBCL treatment?
The recent conditional approval of **BEBT-908** by China’s National Medical Products Administration is not just a milestone for oncology, but potentially a game-changer for adults battling relapsed or refractory diffuse large B-cell lymphoma. With its dual-target mechanism, this first-in-class therapy offers promising efficacy and a well-thought-out access strategy that could reshape treatment standards.
Dive into the full article to discover how BEBT-908 is setting new benchmarks in both clinical outcomes and healthcare affordability.
#SyenzaNews #oncology #pharmaceuticals #MarketAccess