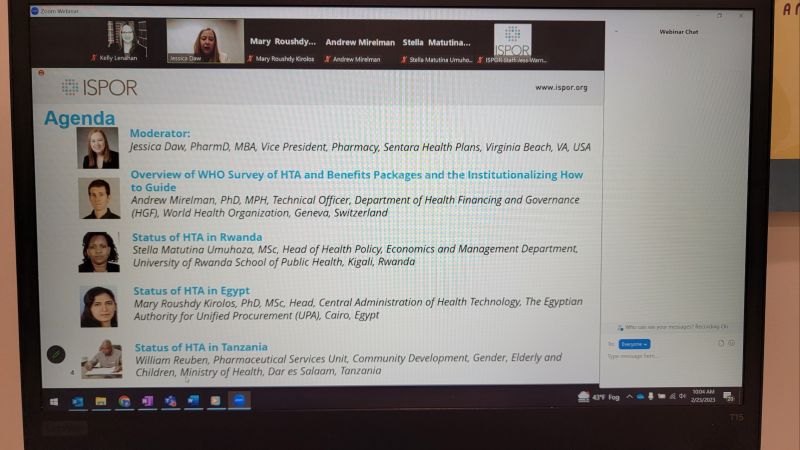
ISPOR HEOR held a Theater focusing on discussions from the ISPOR HTA Roundtable – Middle East and Africa.
Presentations were provided by the World Health Organization, Rwanda School of Public Health, Ministry of Health of Tanzania, and Unified Procurement Authority of Egypt.
Recent Posts
Exploring MFN Policy Effectiveness in Drug Pricing and Innovation
MFN Policy Effectiveness in Countering Freeloading Claims
The MFN policy effectiveness is evident in the coalition letter dated February 12, 2026, which opposes codifying Most-Favored-Nation (MFN) prescription drug pricing b...
Zorginstituut Nederland’s Lecanemab Health Insurance Rejection: Implications for Alzheimer&...
Dutch Health Insurance Rejects Lecanemab Coverage
Zorginstituut Nederland's advisory letter dated 13 February 2026 recommends against including lecanemab (Leqembi®) in the Dutch basic health insurance package, marking a key lecanema...
Revised MenACWY Vaccination Strategy for Adolescents in Germany
G-BA Approves MenACWY Vaccination Strategy Shift for Adolescents
Germany's Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) has approved key amendments to the Protective Vaccination Directive on December 18...