
Introduction
In Saudi Arabia, the healthcare sector is undergoing a profound transformation, aligning with the goals of Saudi Vision 2030. This shift towards a value-based healthcare system requires incorporating the healthcare decision-making with Multi-Criteria Decision Analysis (MCDA) framework to enhance decision processes for orphan drugs and rare diseases.
Establishing a Value-Based Healthcare System
The initiatives under the National Transformational Program 2020 underscore Saudi Arabia’s commitment to restructuring its healthcare sector. By prioritizing patient-centric care and accountability, the country aims to enhance the value proposition of its healthcare services. The establishment of a national healthcare technology assessment (HTA) centre and the integration of e-health facilities are key steps towards achieving this vision.
Challenges and Opportunities in MCDA Implementation
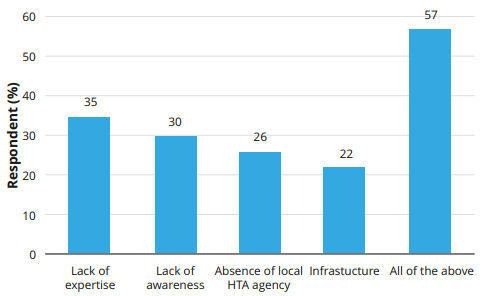
While the benefits of MCDA in healthcare decision-making are globally recognized, Saudi Arabia faces unique challenges in terms of stakeholder awareness and data availability. Overcoming these obstacles requires concerted efforts to enhance expertise, promote stakeholder engagement, and strengthen data infrastructure through initiatives like disease registries. By addressing these challenges, Saudi Arabia can lay a robust foundation for successful MCDA implementation.
Navigating Towards MCDA Integration
The roadmap for integrating MCDA in Saudi Arabia’s healthcare system involves strategic stakeholder mapping, with the Ministry of Health playing a pivotal role in driving the implementation process. Drawing insights from successful models in other countries, Saudi Arabia aims to leverage diverse perspectives from healthcare professionals, payers, and industry stakeholders to ensure the efficacy and relevance of the MCDA framework for orphan drugs and rare diseases.
Future Prospects and Recommendations
As Saudi Arabia embarks on a pilot phase to test the selected criteria and methodology within the MCDA framework, the focus shifts towards evaluating its real-world impact on healthcare decisions. Continuous research, stakeholder collaboration, and refinement efforts will play a crucial role in enhancing the framework’s effectiveness and adaptability to evolving healthcare dynamics. By embracing a culture of ongoing evaluation and dialogue, Saudi Arabia can pave the way for a more informed and value-driven healthcare ecosystem.
Conclusion
The progress in implementing the MCDA framework for orphan drugs and rare diseases in Saudi Arabia represents a significant advancement towards value-based healthcare decision-making. By addressing challenges, encouraging stakeholder engagement, and emphasizing ongoing improvement, Saudi Arabia is on track to transform its healthcare environment through structured and transparent decision-making processes facilitated by MCDA.
