
Introduction
In low- and middle-income countries (LMICs), Health Technology Assessments (HTA) contributes to defining Essential Health Benefit Packages (HBPs). The goal is to achieve Universal Health Coverage (UHC) which means that all persons receive essential health services without financial hardship. This article discusses how HTA can help in developing effective HBPs, dealing with challenges and aligning health priorities based on available resources.
Understanding HTA in Essential Health Benefit Package Design
HTA is a process of evaluating healthcare interventions to determine their value, effectiveness and economic impact. It offers a systematic way for looking at new technologies. On the other hand, designing of HBP entails coming up with comprehensive set of health services that meets the needs of the people within the available funds. While HTA concentrates on single interventions, HBP takes into account entire systems of medicine.
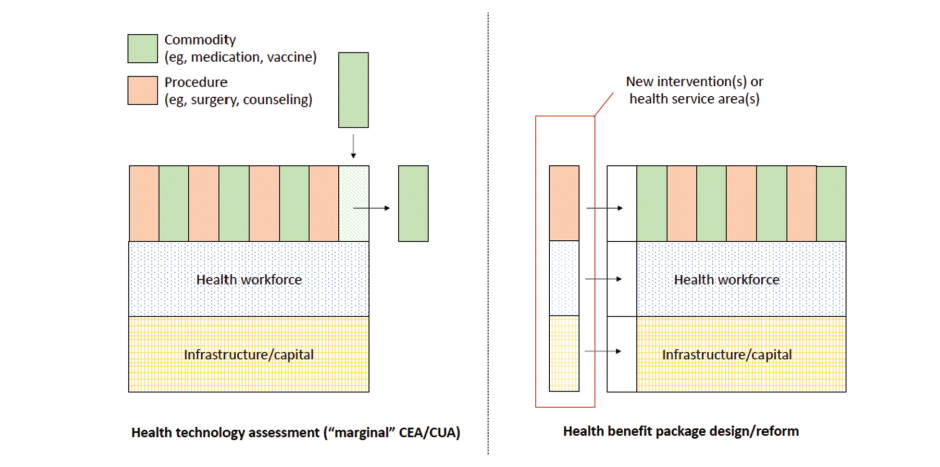
Challenges in LMICs
There are several challenges faced by LMICs when it comes to HTA and HBP owing to inadequate local data as well as technical capacity. In most cases, they tend to use estimates of cost-effectiveness from other contexts which may not be entirely applicable. This dependency may bring about inaccuracies since clinical standards are different among countries while implementation costs vary widely, influenced by many factors including availability of infrastructure etc. Health systems within LMICs are at different stages of development thereby making it difficult for them to adopt holistic HBPs.
A Hybrid Approach: Combining HTA and HBP
To overcome these challenges, it is recommended that a hybrid model be employed which integrates both HTA and HBP. This approach suggests using HTA to inform HBP design through setting specific cost-effectiveness thresholds for different healthcare programs. The method aligns priority setting with organizational concerns and ethical considerations. For example, HTAs can be helpful in deciding whether or not a new drug should be included in the current service mix by comparing its cost-effectiveness against other available treatments.
The Role of the Disease Control Priorities Project
The Disease Control Priorities (DCP) project provides a model for integrating HTA and HBP. DCP3 introduced “extended cost-effectiveness analysis” to incorporate equity and financial protection alongside cost-effectiveness. This approach helps countries tailor HBPs to local contexts by considering multiple dimensions such as health needs, system objectives, and resource availability.
Decision
HTA in Essential Health Benefit Package Design is crucial for designing effective healthcare systems. By adopting a hybrid approach, countries can enhance priority-setting and ensure that health services meet population needs within budget constraints. This method not only improves health outcomes but also promotes equity and financial protection. Strengthening local HTA capacity and aligning it with HBP processes will support the path towards achieving UHC.
