
Introduction
In healthcare valuation, a groundbreaking model has emerged to address the limitations of traditional cost-effectiveness analysis (CEA). Generalised Risk-Adjusted Cost-Effectiveness (GRACE) helps, promoting a patient-centric approach to evaluating healthcare interventions, emphasising individual values and circumstances.
Fixing the Flaws in Traditional Approaches
Current methods of quantifying healthcare intervention value rely heavily on quality-adjusted life-years (QALYs), which fall short in capturing the true value of treatments for severe diseases. Traditional CEA neglects patient perspectives and values, resulting in bias against older, disabled, or complex condition individuals within populations. Therefore, GRACE, a model that puts the context and circumstances of the patient first and transforms the valuation of healthcare.
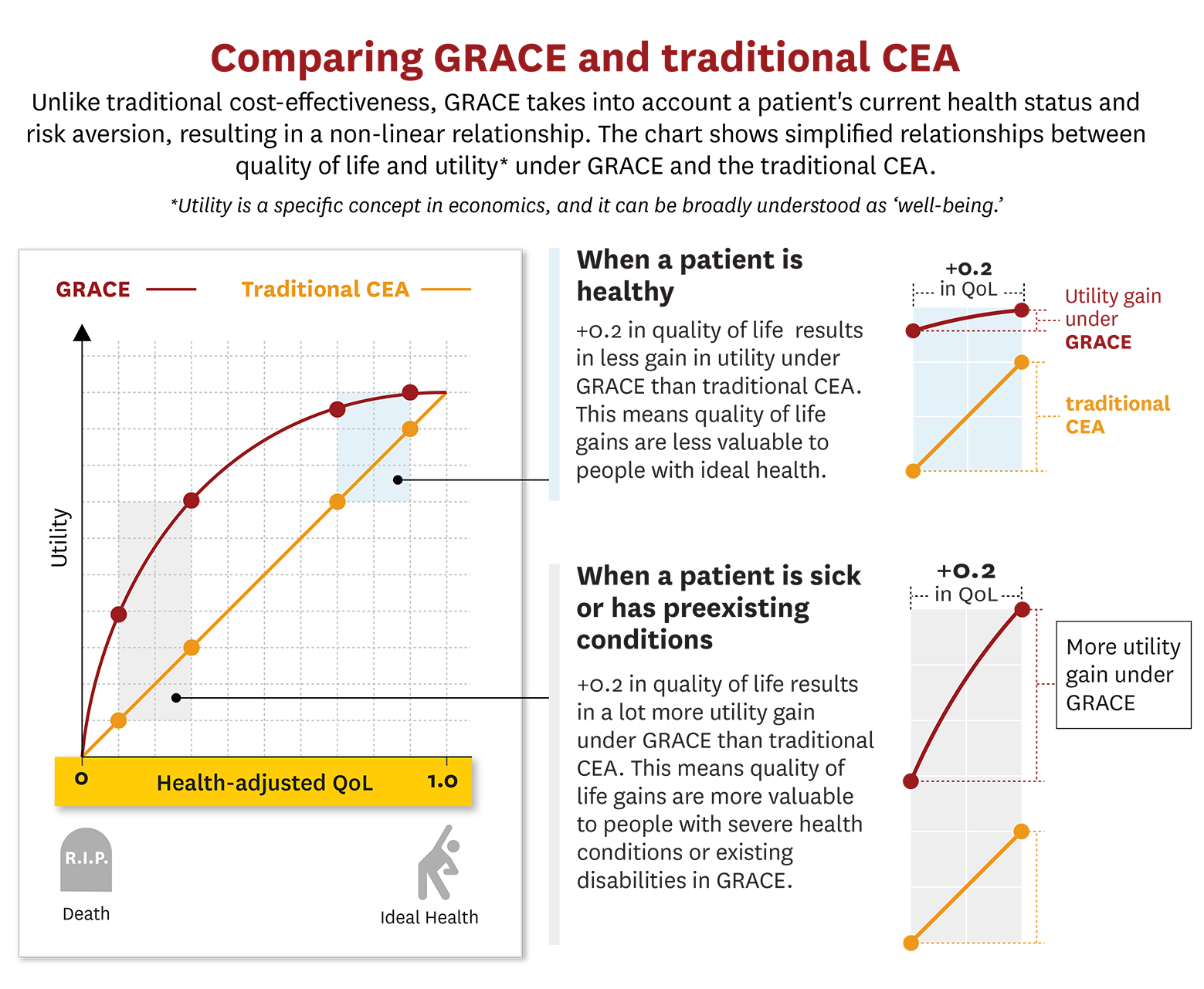
Understanding the Essence of GRACE
GRACE, developed by experts Darius Lakdawalla and Charles Phelps, introduces a patient-centric paradigm in healthcare valuation. GRACE ensures equitable distribution of resources by recognising varying health values based on individual circumstances, benefiting diverse patient groups. This model aligns with the law, promoting fairness and inclusivity in healthcare investments.
Key Features and Implications of GRACE
GRACE transforms valuation by assessing patients’ untreated quality of life and the significance of health enhancements from their viewpoint. GRACE uniquely factors in how a patient’s health status influences their willingness to pay for health improvements, unlike traditional CEA. This nuanced approach results in more generous thresholds for severe illnesses and directs investments where they matter most.
Empowering Patient-Centred Healthcare Decisions
GRACE has the potential to reshape healthcare decision-making in pivotal ways:
- Impact on Coverage Determinations: Adoption of GRACE could lead to increased reimbursement for therapies targeting severe diseases, promoting fairness in resource allocation.
- Reforming Authorisation Rules: GRACE may enhance access to treatments for severely ill individuals by shifting focus from outcome data restrictions to patient preferences.
- Facilitating Shared Decision-Making: Transparency in value assessment empowers patients, clinicians, and health systems to identify high-value therapies tailored to individual needs.
- Encouraging Innovation: By incentivising research and development for treatments addressing severe illnesses, GRACE fosters a culture of patient-centred innovation in healthcare.
Embracing a Patient-Centric Future with GRACE
GRACE marks an evolution in the field of healthcare valuation, bridging the gap between economic principles and patient preferences. By incorporating patient context and values, GRACE offers a comprehensive solution to the shortcomings of traditional valuation methods. GRACE facilitates fairness among varied patients by acknowledging that health value varies with individual circumstances and ensuring equitable resource allocation.
