
Introduction
The domain of medicine stands on the cusp of a significant transformation, spearheaded by the integration of Artificial Intelligence (AI) in medical imaging. This groundbreaking shift promises to enhance diagnostic accuracy, streamline clinical workflows, and ultimately, improve patient outcomes. As we navigate this evolution, understanding the landscape of FDA-cleared AI devices and their application in clinical settings becomes paramount.
The Rise of AI-Enabled CAD in Clinical Practice
The adoption of AI in medicine, particularly in imaging domains such as radiology, is transitioning from development to clinical integration. AI’s role is not just to automate tasks but to enhance the clinician’s ability to diagnose and treat diseases. AI-enabled Computer-Aided Detection (CAD) devices were some of the first to achieve clinical operationalisation. The FDA has approved these devices, which are transforming medical imaging interpretation.
The Clinician-AI Interface
To understand the current state of the clinician-AI interface, a recent study established a database of FDA-cleared AI devices for medical image interpretation. This task is among the first to be clinically operationalised. They discovered 140 FDA clearances from January 2016 to October 2023 for 104 unique AI-enabled CAD products, with some products having multiple clearances over time.
Understanding the Different CAD Types
AI-enabled CAD products are categorised based on their intended use in clinical workflows, each designed to augment the clinician’s capabilities in unique ways. The most common types include:
- Computer-Aided Triage (CADt): These devices prioritise suspicious cases for review, flagging them with a binary indicator. This early detection mechanism aims to expedite the clinician’s review process.
- Computer-Aided Detection (CADe): CADe devices assist in locating lesions by overlaying markings on images, enhancing the detection process.
- Computer-Aided Detection and Diagnosis (CADe/x): These devices offer a more granular analysis by assigning scores to lesions or cases, aiding both detection and diagnosis.
- Computer-Aided Diagnosis (CADx): Focusing on diagnosis, CADx devices provide insights without marking lesion locations, supporting clinicians in interpreting exams.
- Computer-Aided Automatic Interpretation (CADa): A novel category, CADa devices automatically interpret exams for specific applications without clinician review.
CAD in Early Disease Detection: A Focus on Breast Cancer and Intracranial Haemorrhage
Among the various types of CAD applications, Computer-Aided Triage (CADt) has emerged as the most prevalent, representing a significant portion of the market. This predominance is attributed to its critical role in flagging suspicious cases for prioritised review, thereby accelerating the diagnostic process. Interestingly, CADt devices have found their most frequent application in the fields of breast cancer and intracranial haemorrhage (ICH) detection. These areas benefit immensely from the early detection capabilities of CADt, where timely intervention can dramatically influence patient outcomes. For instance, in breast cancer, the early identification of suspicious lesions can lead to quicker diagnostic follow-up and treatment planning. Similarly, in the case of ICH, rapid detection is crucial for the management and treatment of this potentially life-threatening condition. The widespread adoption of CADt in these domains underscores the significant impact AI can have on managing diseases with high stakes for early detection.
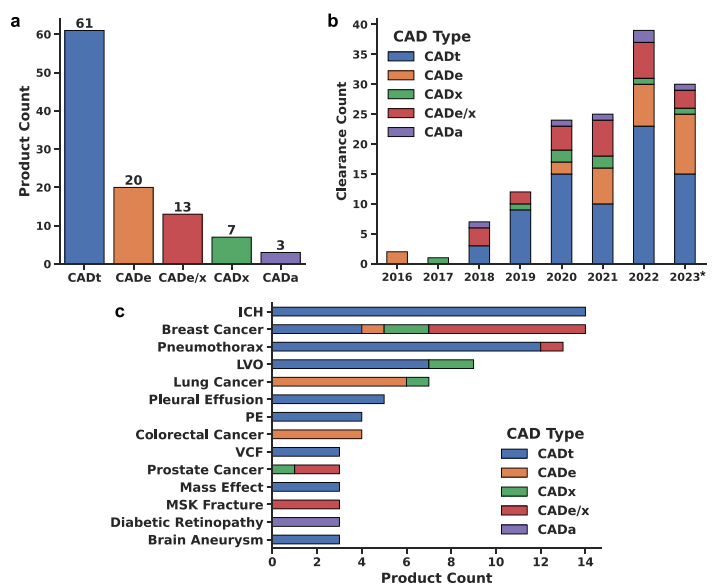
*ICH= intracranial hemorrhage; LVO= large vessel occlusion; PE= pulmonary embolism; VCF= vertebral compression fracture; MSK= musculoskeletal
The Clinician-AI Interface: Outputs and Explainability
A pivotal aspect of integrating AI into clinical workflows is the nature of the outputs generated by these devices. Outputs serve two primary functions: conveying the core prediction of the AI model and supporting this prediction to enhance understanding and trust among clinicians. The latter is achieved through various forms of explainability, such as localisation of findings, exemplar-based explanations, and counterfactual approaches. These methods aim to demystify the AI’s decision-making process, fostering a deeper trust and reliance on AI-assisted diagnostics.
The Path Forward: Integration and Clinical Efficacy
The integration of AI in medical imaging is not without its challenges. Different diseases and clinical settings demand unique integration strategies due to the variability in AI outputs and the diversity of CAD types. Recent FDA communications emphasise the need to know the limitations and intended use of CADt devices. This is especially important for critical areas such as detecting intracranial large vessel occlusion. As AI becomes more prevalent in healthcare, we must maintain a focus on improving clinical efficacy. This requires careful integration and clinician training. By doing this, we can ensure that AI not only supports but enhances clinical decision-making. This will lead to better patient care and outcomes.
Conclusion
The integration of AI into medical imaging represents a significant leap forward in the pursuit of advanced diagnostic tools and enhanced patient care. By understanding the nuances of FDA-cleared AI devices and their role in clinical workflows, healthcare professionals can harness the full potential of this technology. As we move forward, the synergy between clinicians and AI will undoubtedly shape the future of medicine, making it more accurate, efficient, and patient-centric.
