
The Inflation Reduction Act (IRA) has initiated a new era in drug pricing. Soon, the Centers for Medicare & Medicaid Services (CMS) in the U.S will negotiate prices for certain high-cost drugs. This includes direct-acting oral anticoagulants (DOACs) apixaban (Eliquis) and rivaroxaban (Xarelto). These were among the first 10 drugs selected for negotiation.
Clinical and Economic Comparisons
The Institute for Clinical and Economic Review (ICER) released a Special Report that examines these two drugs, primarily used for non-valvular atrial fibrillation (NVAF). They were compared to warfarin, the older standard therapy, and dabigatran, the first DOAC available as a generic medication. The study considered patient experiences, clinical effectiveness, and cost comparisons.
The findings indicate that DOACs improve outcomes for NVAF patients compared to warfarin. Both apixaban and rivaroxaban have shown small net benefits compared to warfarin. However, when compared to dabigatran, the benefits are comparable or small.
Considering the Pricing Aspect
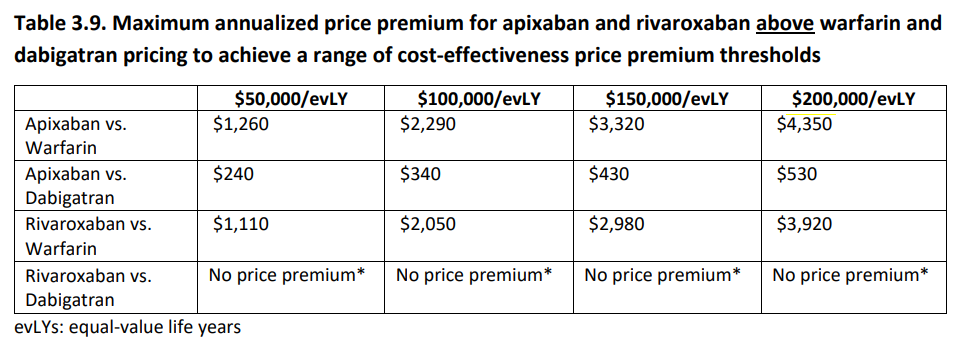
To inform drug price negotiations, they have calculated price premiums at various cost-effectiveness thresholds for apixaban and rivaroxaban relative to warfarin and dabigatran. For instance, the annual price premium for apixaban relative to warfarin ranges from $1,260 at a threshold of $50,000/evLYG to $4,350 at $200,000/evLYG. However, rivaroxaban was not associated with health gains compared to dabigatran. Thus, not supporting a price premium above CMS pricing for dabigatran.
This information provides a foundation for CMS and other stakeholders to make informed decisions about the value and pricing of these important drugs.
