
Introduction
Respiratory syncytial virus (RSV) is the causative agent of a highly contagious lower respiratory tract infection that continues to pose a significant health risk worldwide. Infection can lead to bronchitis and pneumonia, and severe disease often occurs in young children and infants. Moreover, RSV is the leading cause of infant hospitalisation, and is more likely to be fatal in premature infants and those with underlying medical conditions.
The Burden of RSV in Colombia
In Colombia, the healthcare system struggles to accommodate all patients needing neonatal intensive care units (NICUs). The high costs of expanding NICUs make it difficult to meet the demand. Consequently, prioritising the most severely ill patients becomes essential. This study explores the cost-effective strategy of using palivizumab to prevent RSV-related hospitalisations in three population groups: preterm neonates (≤ 35 weeks gestational age), infants with bronchopulmonary dysplasia (BPD), and infants with hemodynamically significant congenital heart disease (CHD).
Palivizumab: A Preventive Measure
Palivizumab is an immunoprophylaxis that reduces the risk of RSV-related hospitalisations. Clinical studies show that prophylactic use of palivizumab can lower the risk of hospitalisation by 55% for high-risk infants. By preventing severe RSV infections, this drug helps free up NICU beds for other critical cases. This strategy not only saves costs but also improves the quality of life for infants and their families.
Economic Evaluation of Palivizumab
The study used a decision tree model comparing costs and outcomes of using palivizumab versus not using it (Figure 1).
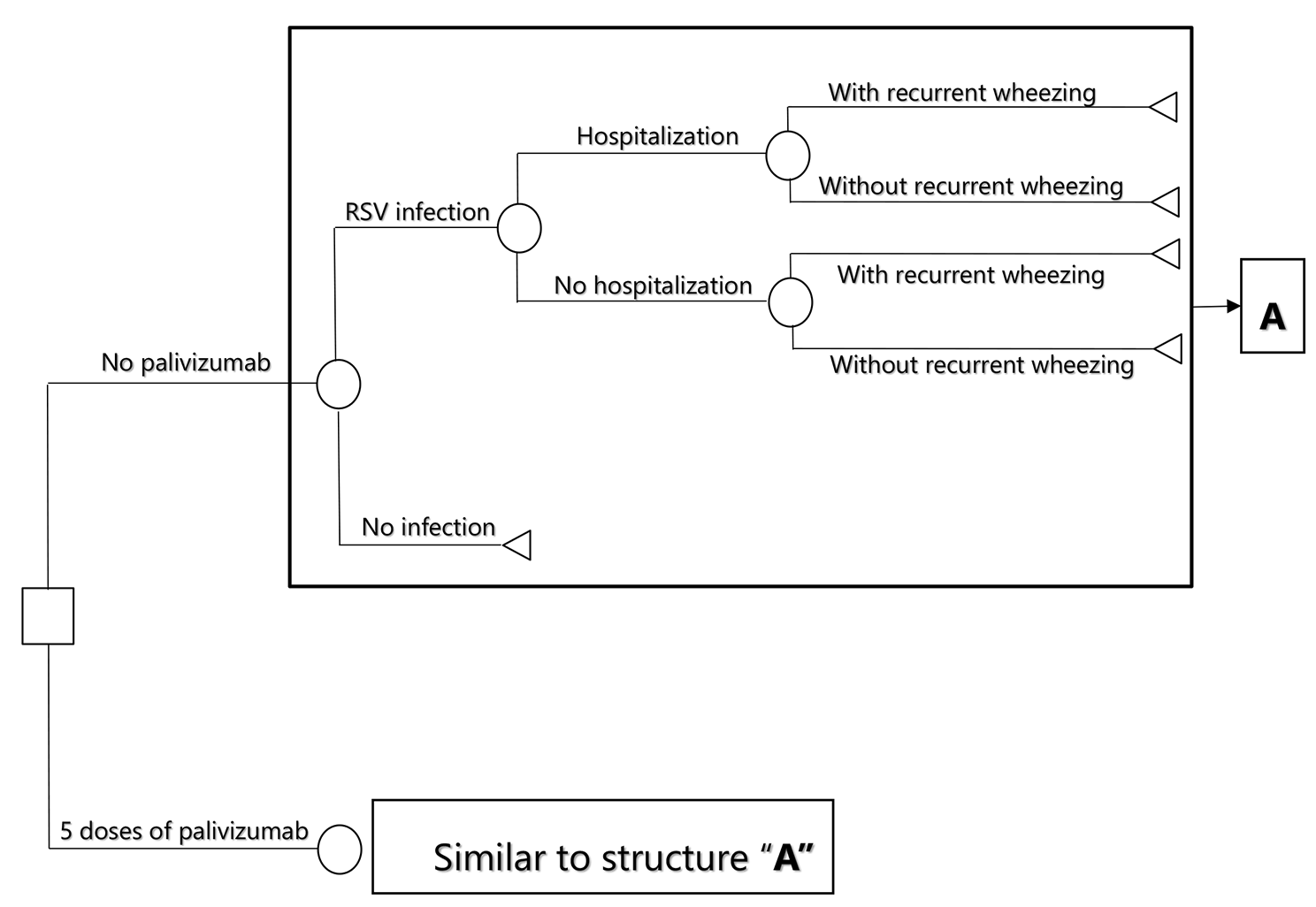
Figure 1. Decision tree model structure with two health states for prophylaxis with palivizumab to prevent the respiratory syncytial virus in children
Each hospitalisation costs between $2,076 and $3,772, and the average price of each relapse is $174. Each patient experiences an average of 4.63 relapses per year for the first six years, resulting in an average saving of $4,834 per patient. While $174 per relapse may not significantly impact the Colombian health system, $4,834 represents substantial savings, especially in low- and middle-income countries. Moreover, prevention of wheezing exacerbations improves quality of life for these patients.
The likelihood that palivizumab is an effective treatment for preventing RSV infection compared to a placebo, given a willingness to pay of 1 GDP per capita in Colombia, is 95.4% for preterm neonates ≤ 35 weeks gestational age (wGA), 56.1% for infants with BPD, and 84.2% for infants with CHD.
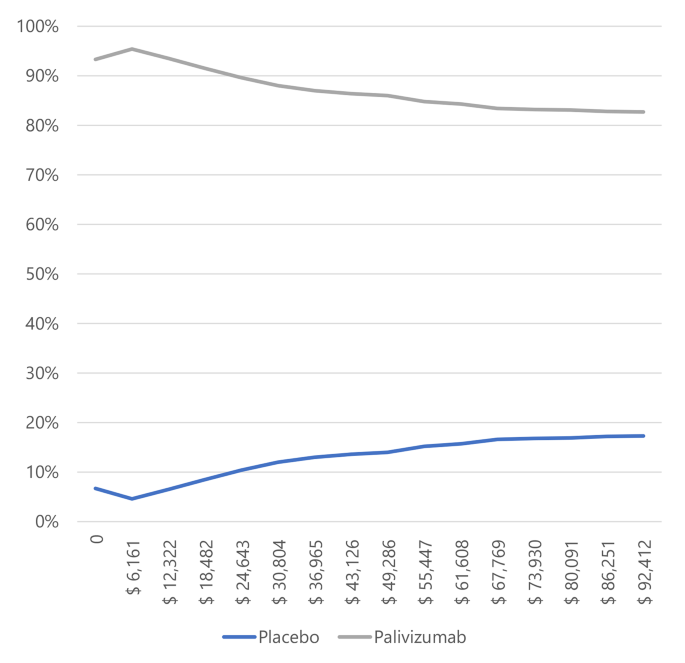
Figure 2. Willingness to pay curves to obtain the Maximum Net Benefit of palivizumab vs. placebo in preterm neonates ≤ 35 weeks gestational age in Colombia, 2022
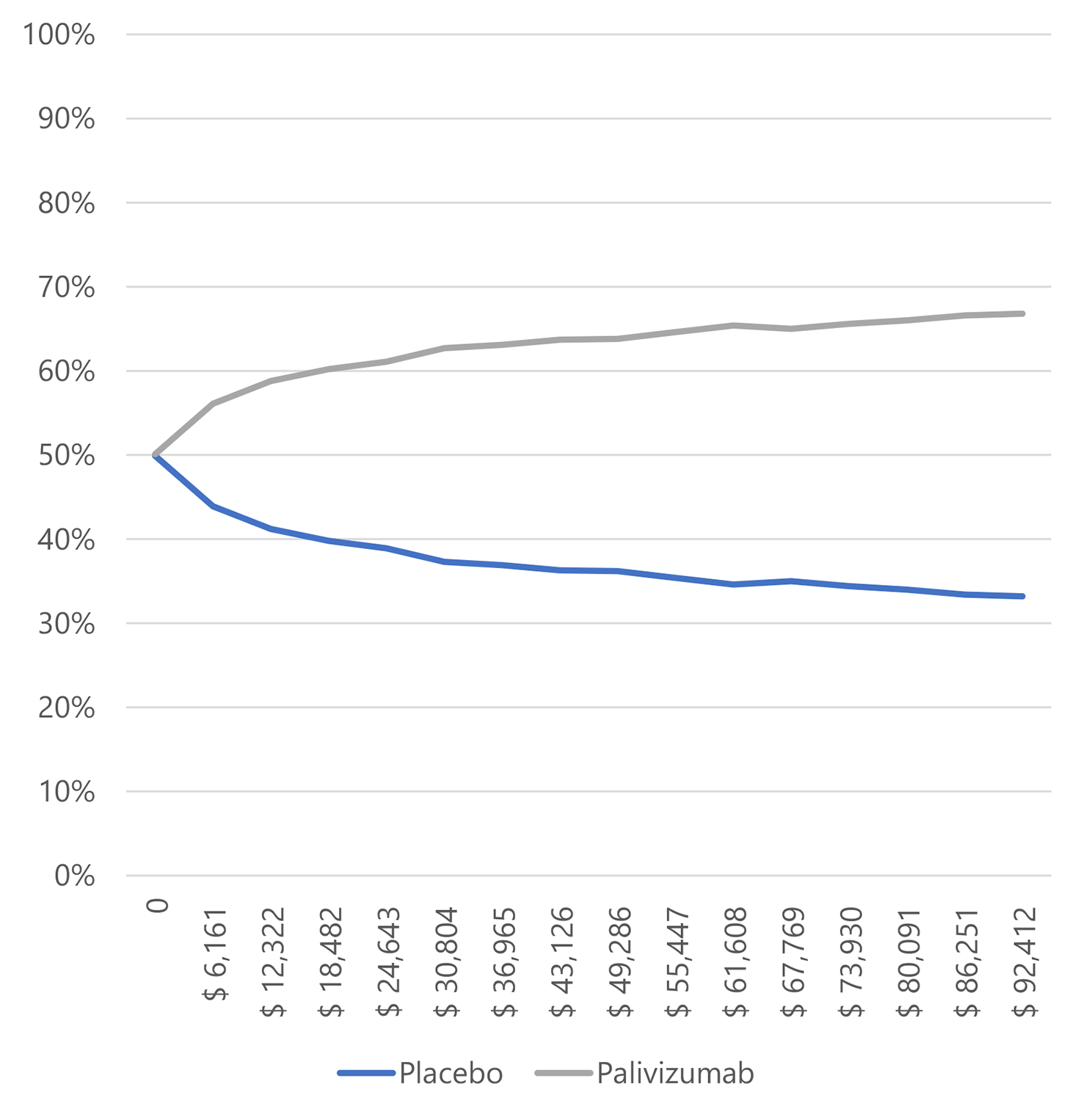
Figure 3. Willingness to pay curves to obtain the Maximum Net Benefit of palivizumab vs. placebo in infants with bronchopulmonary dysplasia in Colombia, 2022.
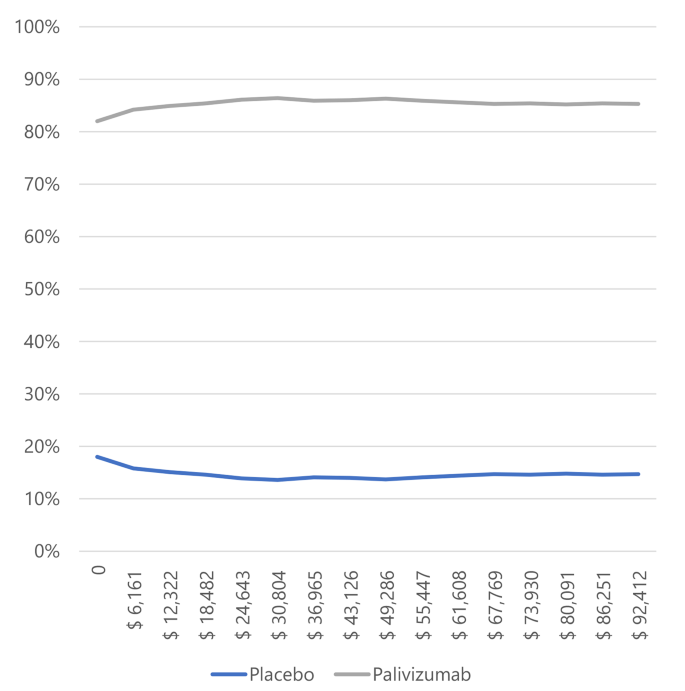
Figure 4. Willingness to pay curves to obtain the Maximum Net Benefit of palivizumab vs. placebo in infants with congenital heart disease in Colombia, 2022.
Key Findings and Implications
Palivizumab emerged as the dominant cost-saving strategy for preventing RSV-related hospitalisations in pre-term neonates ≤ 35 wGA, infants with BPD, and infants with CHD. It also highlighted the importance of considering indirect costs, such as lost productivity of caregivers. By reducing hospitalisations, palivizumab allows the healthcare system to allocate resources more efficiently and treat other non-preventable diseases.
Conclusion
Implementing cost-saving strategies in healthcare can significantly impact patient outcomes and resource allocation. Preventive measures like palivizumab can improve care and reduce costs in Colombia’s challenged healthcare system.
