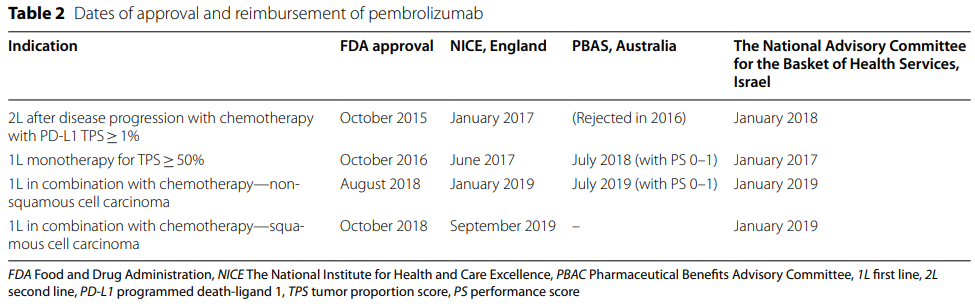
🌍 Are we on the brink of a new era in Hepatitis C treatment?
Atea Pharmaceuticals is hosting a virtual KOL panel on May 14, 2025, featuring top experts discussing the challenges faced by HCV patients and sharing insights from the promising results of their Phase 2 study on bemnifosbuvir and ruzasvir. This could be a game-changer in advancing HCV treatments through ongoing Phase 3 trials.
Don’t miss out on how these developments might reshape the future landscape for HCV patients! Click to read more about the panel and the innovative therapies in the pipeline.
#SyenzaNews #biotechnology #HealthEconomics