Introduction:
New cancer therapies, such as pembrolizumab, come with high costs, presenting a unique challenge for resource allocation decisions across nations and healthcare systems. Developed countries with national universal health insurance coverage have established different methods. Time to Reimbursement of Pembrolizumab and other oncology treatment are of utmost importance for high level of care. These methods orderly and transparently assess new technologies before adding them to their list of reimbursed services. This process, known as the “fourth hurdle”, is a stage after a country registers the technology. Here, the new technology must show value for money. It also needs to prove its effectiveness and safety before it can qualify for reimbursement from public funds.
International Approaches:
This research utilises a comparative approach, examining the reimbursement process for pembrolizumab, a cancer treatment drug, in three different healthcare systems: The National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK), the Pharmaceutical Benefits Advisory Committee (PBAC) in Australia, and the National Advisory Committee for the Basket of Health Services in Israel. Data were collected from publicly available websites and relevant literature.
Time to Reimbursement of Pembrolizumab:
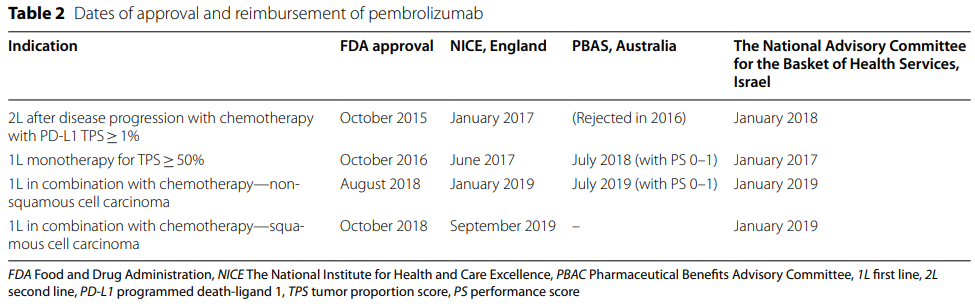
The research found significant differences in time to reimbursement in these countries, with Israel approving two conditions earlier than the UK and Australia. This paper suggests that unique healthcare policies and systems in these countries could account for these differences.
Conclusion:
The study concludes by suggesting the need for a debate on how to encourage earlier access to necessary and life-saving cancer treatments in countries where the reimbursement process is longer. The most important challenge was budget limitations among many factors that are taken into account in the reimbursement process, and length of time from registration to reimbursement in the public health system of each country.