Introduction:
A transformative innovation in paediatric cardiac care is making waves: the Autus Valve. This pioneering paediatric pulmonary valve replacement promises to significantly reduce the need for invasive surgeries in children with congenital heart conditions.
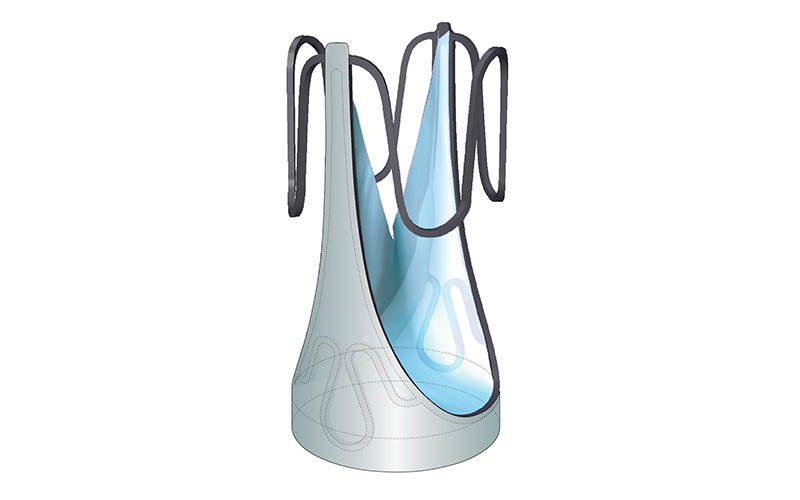
The Autus Valve: A Leap Forward in Pediatric Cardiology
The Autus Valve stands as a glimmer of hope for approximately 110,000 children in the U.S. suffering from congenital pulmonary valve disease. Traditional prosthetic valves, unsuitable for growing bodies, necessitate multiple surgeries throughout childhood. The Autus Valve, however, is designed to expand in tandem with a child’s growth, potentially postponing the need for replacement until adulthood.
A Collaborative Triumph in Medical Engineering
The conception of the Autus Valve is a testament to the collaborative spirit of the medical community. Born from the innovative minds at Boston Children’s Hospital and further developed in conjunction with MIT. This valve is a marvel of medical engineering, combining proven materials with a novel design inspired by human venous valves.
Minimally-Invasive Adjustments: A New Era of Treatment
The Autus Valve’s design allows for non-invasive adjustments through transcatheter balloon dilation. This minimally-invasive approach is a game-changer, offering a swift recovery and sparing young patients from the burdens of repeated surgeries.
Ensuring a Brighter Future for Children
The FDA-approved clinical trials for the Autus Valve, currently underway, mark a significant milestone in paediatric cardiac care. With the potential to offer a seamless transition through childhood and into adult life, the Autus Valve not only represents a medical innovation but a pathway to improved quality of life for children worldwide.
Conclusion:
The Autus Valve is more than a medical device; it’s a symbol of hope for families and a testament to the relentless pursuit of better healthcare solutions. As we await the results of ongoing clinical trials, the anticipation within the medical community is palpable. This pioneering technology could redefine the landscape of paediatric cardiac care. Therefore, offering children with congenital heart conditions the chance to live fuller, healthier lives. Boston Children’s is now conducting US FDA-approved early clinical studies to examine the valve’s effectiveness in children aged between 2 to 11 years.
