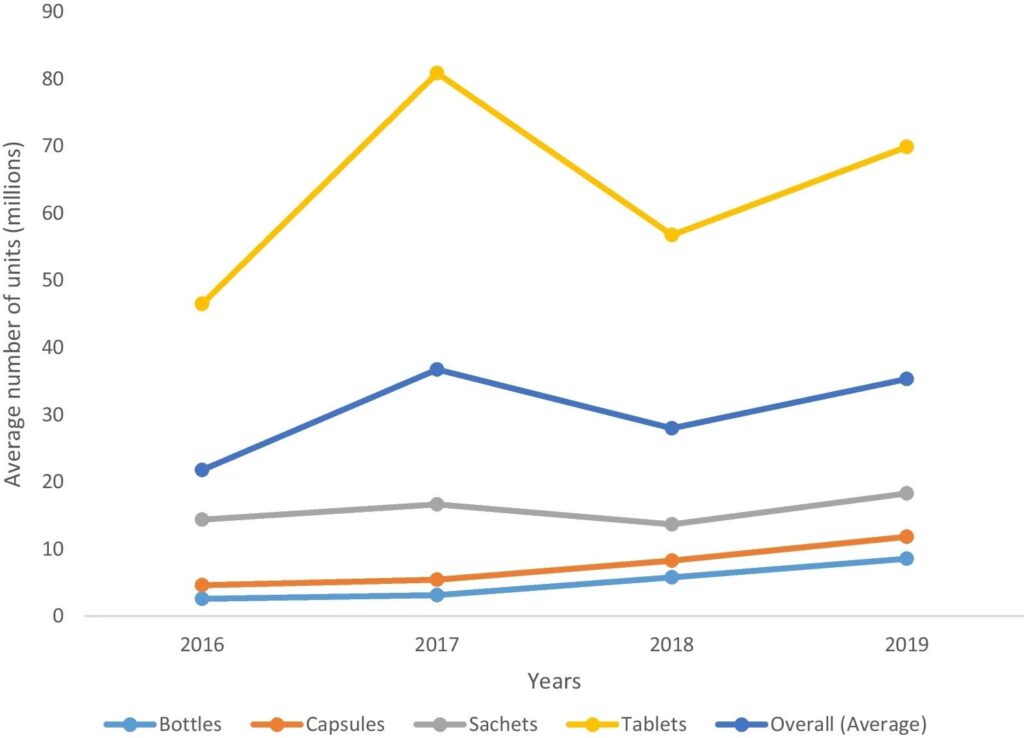
The study examines the impact of import verification fees on local pharmaceutical production in Uganda. The findings suggest that the policy has been effective in promoting local production, with an 8.2% increase in production capacity observed. The study also highlights the challenges faced by the local industry, including limited access to raw materials, high operating costs, and inadequate quality control equipment. The authors recommend that the government provide necessary support to the local industry, including policies to protect local manufacturers from high taxation and promote local production of raw materials.
Recent Posts
Pharmaceutical Tender Investigation Sparks Regulatory Scrutiny in South Africa
Pharmaceutical Tender Investigation Targets Ascendis, Pharma Q, and Sonke
The Competition Commission of South Africa has launched a pharmaceutical tender investigation into manufacturers Ascendis, Pharma Q, and...
South Africa Health Reform: Navigating Innovations and Challenges for 2026
South Africa Health Reform: Contrasts in 2026 Public Health Agenda
South Africa health reform defines the 2026 public health landscape as a mix of breakthroughs like HIV prevention injections and AI-driven TB diagnos...
Enhancing Patient Safety in Africa’s Healthcare Transformation
Prioritizing Patient Safety Africa in 2026 Trends
Patient safety Africa stands at the forefront as regulatory bodies drive healthcare self-reliance amid global shifts. A recent article (linked below) outlines five pivotal trends pro...