
Introduction
The American healthcare landscape is witnessing a significant shift with the introduction of biosimilars, designed to create market competition and reduce the financial burden of biologic medications. In 2021, patients and payers in the US spent over $500 billion on prescription drugs, with biologics representing a substantial portion of these costs. This article examines the impact of biosimilar competition on out-of-pocket (OOP) costs for patients using biologics including filgrastim, infliximab, pegfilgrastim, epoetin alfa, bevacizumab, rituximab, and trastuzumab.
Understanding Biosimilars
Biosimilars are analogous to generic drugs but for complex biologic medications. The rationale for their introduction is to provide a cost-effective alternative to expensive brand-name biologics, once patent protections expire. With prices typically 15% to 35% lower, biosimilars are poised to deliver significant savings to the healthcare system. In January 2021, there was just one biosimilar available for epoetin alfa, whereas the other six biologics had a minimum of two biosimilars, with trastuzumab having the highest number of five.
The Cost Conundrum
Infliximab accounted for 31% of patient-years, making it the most often utilised biologic. Conversely, epoetin alfa constituted a mere 3% of patient-years, rendering it the least commonly employed. Despite the potential for reduced costs, the real-world impact on individual patient OOP expenses remains ambiguous. Insurance benefit designs, reimbursement rates, and the timing of deductible fulfilment all contribute to the complexity of assessing biosimilar competition’s direct effect on patient costs.
The Study’s Findings
A recent analysis focused on commercially insured patients using seven clinician-administered biologics with available biosimilars. The results revealed a trend towards increased OOP costs post-biosimilar introduction, challenging the assumption that biosimilars would automatically lead to lower patient expenses.
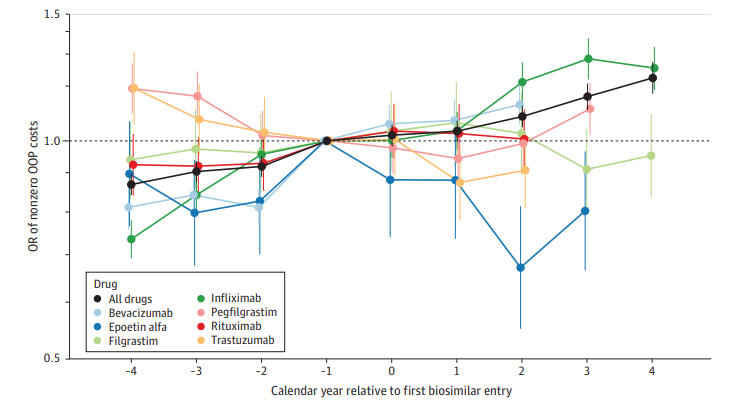
Before and After Biosimilar Availability
Variations in Out-of-Pocket Costs
The study highlighted that the influence of biosimilar competition on OOP costs is not uniform across all biologics. While some drugs showed a decrease in OOP expenses following biosimilar entry, others exhibited an increase or no significant change, underscoring the complexity of the issue. Claims for biosimilar infliximab had higher OOP costs than the reference biologic (AOR, 1.22), while claims for bevacizumab showed the opposite tendency (AOR, 0.77).
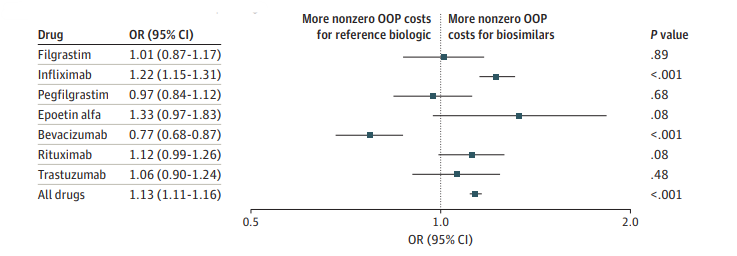
The Disconnect Between Savings and Patient Costs
Despite biosimilars generating nearly $13 billion in savings, these have not translated into lower OOP costs for patients. The study suggests that while biosimilar competition may reduce overall healthcare spending and potentially lower premiums, these benefits do not directly impact individual patient costs.
Biosimilar Competition and Patient Costs
Previous biosimilar-OOP cost studies were inconsistent. Filgrastim found that biosimilar introduction lowered monthly OOP costs only for high-deductible plans. Infliximab biosimilar trials showed a minor decrease in OOP expenses per claim, but higher annual spending owing to cost-sharing and lack of discounts. Biosimilar users had decreased OOP costs per cycle, according to pegfilgrastim studies. The findings underscore the need for regulatory measures to ensure that biosimilar savings enhance patient affordability and access. Policymakers could consider laws to limit OOP expenses for biosimilars or decouple patient cost-sharing from inflated reimbursement rates.
Conclusion
The introduction of biosimilars represents a promising strategy to contain healthcare costs. However, this article indicates that, thus far, biosimilar competition has not consistently lowered the OOP costs for patients using biologics. It is imperative for policymakers to implement strategies that ensure the economic benefits of biosimilars extend to patients, enhancing affordability and access to essential medications.
