
Introduction:
The World Health Organisation (WHO) has issued a global mandate to eliminate cervical cancer as a public health concern (i.e., reach a rate of less than 4 cases per 100,000 woman-years). This demands rigorous evaluation of country-specific cervical cancer control programmes. The term ‘elimination as a public health problem’ is defined as “achieving the measurable global targets set by the World Health Organisation for a specific disease, based on population data”.
Understanding Cervical Cancer Elimination:
Cervical cancer develops over a long period post the acquisition of a human papillomavirus (HPV) infection. Mathematical simulation modelling has proven to be an effective tool in understanding the burden of cervical cancer under past and future prevention efforts. These models have been instrumental in planning WHO’s elimination goals in numerous countries including Australia, the United States (US).
The Role of HPV Vaccination:
HPV vaccination plays a crucial role in achieving cervical cancer elimination in the long term. However, scaling up cervical screening expedites the timing of elimination. In settings with limited treatment access, scaling up cervical cancer treatment plays an additional, crucial role in saving lives in the short term.
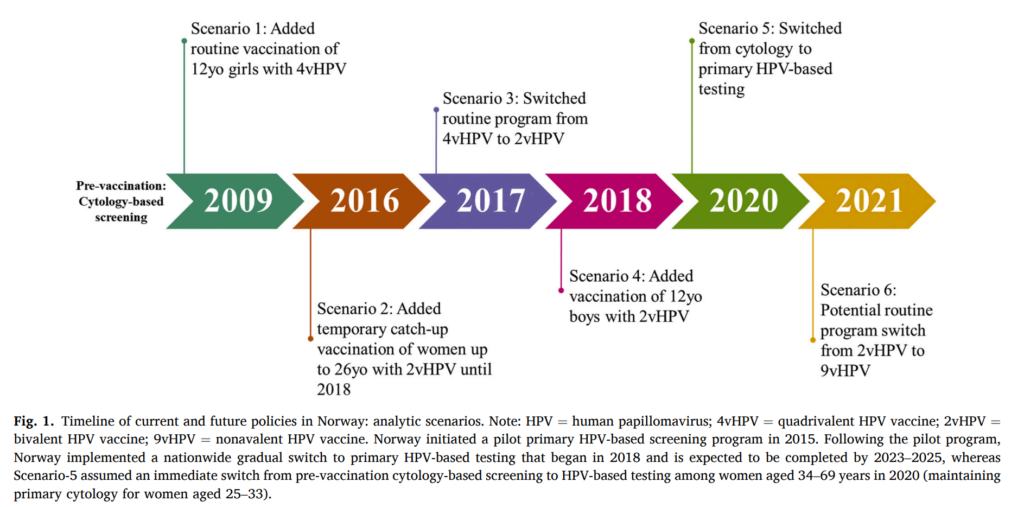
The Case of Norway:
Norway, similar to other high-income countries, has considerably reduced cervical cancer incidence through a long-standing organised cytology-based screening programme. The 2009 implementation of routine girls-only adolescent HPV vaccination program has achieved high coverage. This can lead to Norway having the potential to eliminate cervical cancer in the near future.
The Impact of Screening and Vaccination Policies:
One study aimed to assess how the HPV vaccination and cervical cancer screening policy decisions in Norway over the last decade have influenced the timing of cervical cancer elimination. As the value of implementing a new policy is an explicit component of priority-setting in Norway, they also evaluated the cost-effectiveness of a potential switch of the routine HPV vaccination program from bivalent HPV vaccine (2vHPV) to the nonavalent HPV vaccine (9vHPV).
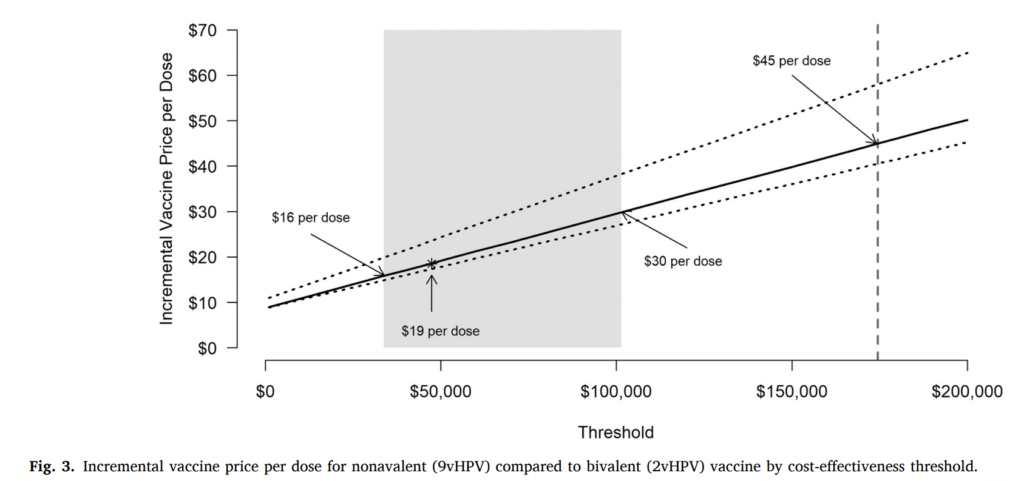
Conclusion:
Based on this study, it is projected that Norway could aim towards eliminating cervical cancer by 2039. Over the last decade, Norway has implemented cervical cancer control policies such as routine HPV vaccination and primary HPV-based testing. These measures may have hastened the elimination timeframe by over 17 years. Addition to this, they could prevent more than 23,800 cases by 2110. A potential switch to 9vHPV may lead to greater benefits, but does not affect elimination timing and it would not be considered cost-effective to switch to the nonavalent vaccine in Norway unless the incremental cost per dose was $19 or less compared to 2vHPV.
