
Introduction
The Global Burden of Disease (GBD) study has highlighted the increasing prevalence of musculoskeletal disorders in Africa, significantly affecting children and young people (CYP). This burden is exacerbated by delayed access to healthcare and the use of prolonged corticosteroids due to the unavailability of appropriate medicines. The World Health Organization (WHO) Essential Medicines List (EML) and the WHO EML for children (EMLc) are crucial frameworks to address these challenges. The alignment of National Essential Medicines Lists (NEMLs) in African countries with the WHO EML and EMLc, is a crucial step for rheumatic diseases in CYP.
The Global Burden of Rheumatic Diseases in CYP
Rheumatic diseases in children are a major source of pain and disability. This often affects children during their prime educational and career-defining years. The chronic nature of these diseases means that CYP require consistent and effective treatment to maintain their quality of life. However, in many African countries, the healthcare infrastructure is not equipped to provide timely and adequate care. Factors such as low gross domestic product, prioritisation of communicable diseases, and lack of healthcare funding further compound the issue.
WHO Essential Medicines List: A Crucial Framework
The WHO EML and EMLc are evidence-based policy documents that streamline access to essential medicines. Since its inception in 1977, the WHO EML has expanded to include over 500 medicines. The list is revised every two years to ensure it remains up-to-date and effective. In 2007, the WHO EMLc was developed to address the unique health needs of the paediatric population. It presents the minimum medicines needed for priority conditions to enable a functional basic healthcare system in each country. These lists also serve as a benchmark for countries to develop their NEMLs, which are essential for establishing Universal Health Coverage.
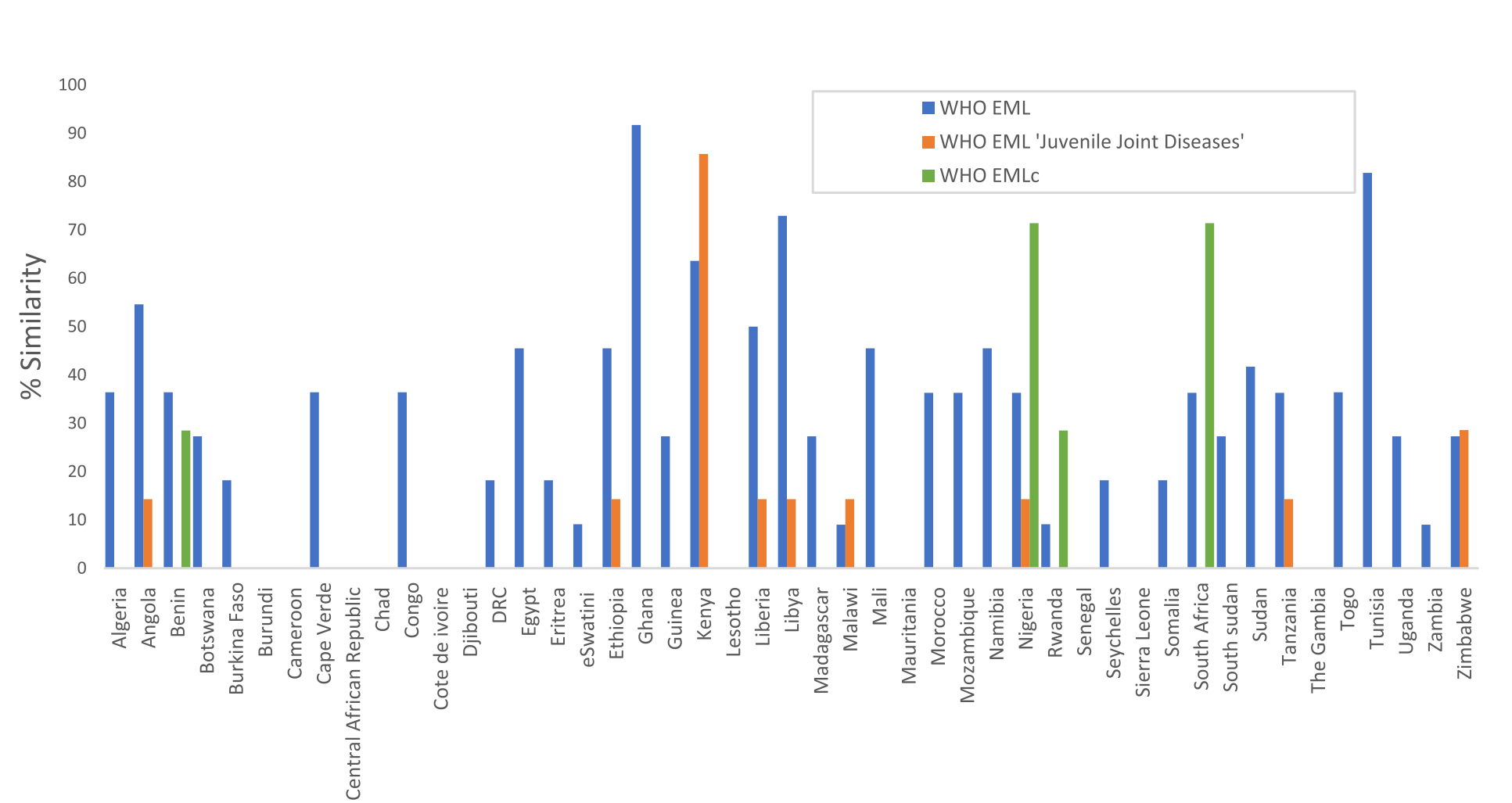
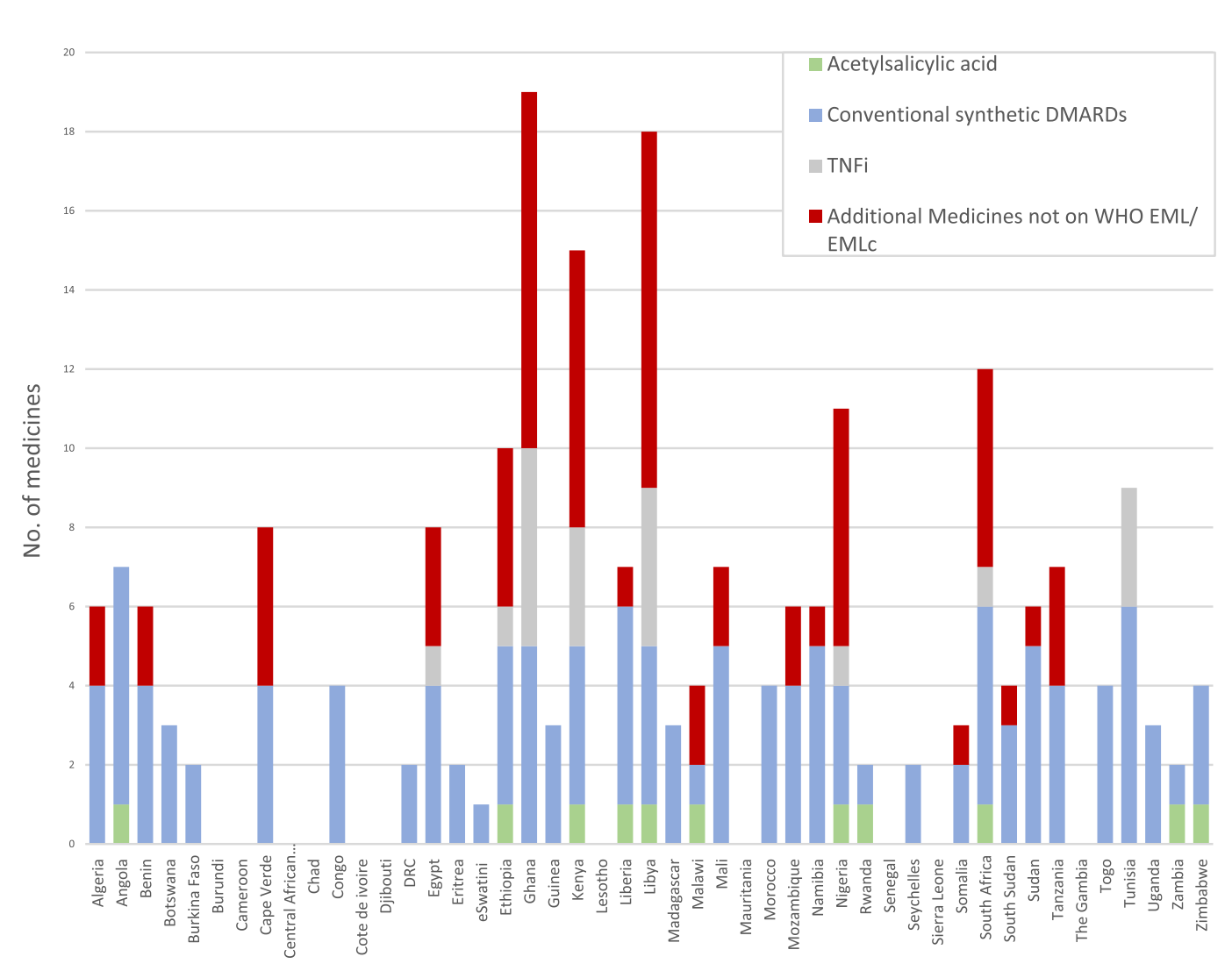
Current Status of NEMLs in Africa
Despite the importance of NEMLs, only four African countries have updated their lists since 2021. The Global Paediatric Musculoskeletal Task Force (TF), a virtual community involved in paediatric rheumatology care, has pinpointed a problem. Lower income countries lack access to medicines for treating rheumatic diseases in children and young people (CYP). Most countries have a 50% or lower similarity with the 2021 WHO EML. Conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) are more commonly available, presumably due to their utility in adult rheumatic diseases. However, only eight countries (South Africa, Ethiopia, Ghana, Nigeria, Kenya, Libya, Tunisia and Egypt) list biologic DMARDs. These serve as a reflection of modern treatment approaches. The availability of these medicines is crucial for improving outcomes in CYP with rheumatic diseases.
Strategies for Improvement
Policymakers need to prioritise musculoskeletal disorders and support rheumatology services. The Global Strategy for Musculoskeletal Health offers a framework that African countries can adapt. Programs like the ‘Non-Communicable Diseases Prevention and Control Programme’ in Tanzania, which integrates the treatment of chronic diseases with existing healthcare services, can serve as models.
Innovative financing, such as managed entry agreements and the use of the Medicines Patent Pool, can reduce the cost of medicines. The successful implementation of a universal access program for JIA in Chile highlights the benefits of government-mandated policies. External funding support such as the United States President’s Emergency Fund for Aids Relief (PEPFAR) and the Global Fund, can help to provide cost-effective care in lower income countries.
Adequate training for healthcare providers and the integration of telemedicine can improve the diagnosis and treatment of rheumatic diseases in CYP. The ‘Practical Approach and Care Kit for children’ in the Western Cape region of South Africa now includes an extensive section on musculoskeletal disorders and inflammatory arthritis. This manual serves as a primary care tool in the country.
Conclusion
Improving access to essential medicines for rheumatic diseases in CYP is a multifaceted challenge. It will require coordinated efforts at the policy, healthcare, and community levels. By aligning NEMLs with the WHO EML and implementing holistic care models, African countries can significantly improve the quality of life for CYP with rheumatic diseases
