
Introduction:
Atopic dermatitis (AD) presents a significant health challenge, impacting about 10% of Japan’s population across all age brackets. Notably, 60% of cases originate in childhood, with 10%–20% surge in prevalence in developed nations over the past 30 years. Atopic dermatitis causes physical discomfort, disrupts sleep, and impairs psychosocial well-being, severely affecting quality of life. Adult AD patients in Japan incur a significant annual cost of illness, totaling 3 trillion JPY. Studies show employed adults with AD in Japan face 30% to 34% work impairment, highlighting impact on individuals and society. Abrocitinib approval in Japan offers hope for moderate-to-severe AD, promising improved outcomes and easing societal and economic burdens.
Treatment Landscape in Japan:
In Japan, the treatment landscape for moderate-to-severe atopic dermatitis (AD) has seen notable advancements in recent years. In 2018, the introduction of Dupilumab marked a milestone as the first systemic treatment targeting the anti-interleukin (IL)-4 receptor α, revolutionising AD management. Subsequent developments included the expansion of authorised indications for baricitinib, an oral Janus kinase inhibitor (JAKi), to encompass AD in 2020. The approval of upadacitinib and abrocitinib in 2021 further diversified treatment options, both being oral JAK inhibitors. Notably, abrocitinib, a JAK1-selective inhibitor, stands out as the newest addition to the approved JAK inhibitor lineup. Approved by the Japanese Ministry of Health, Labour and Welfare (MHLW), it addresses AD effectively. This evolving landscape highlights a shift towards using interleukins and JAK inhibitors. This trend reflects a growing emphasis on targeted therapies in Japan’s approach to treating dermatological conditions.
Economic Evaluation and Methodology:
Given the rising medical costs in Japan, a cost-effectiveness analysis (CEA) is crucial to assess the value of introducing abrocitinib into the healthcare system. CEAs provide insights into indirect costs like productivity losses, particularly significant in AD cases. The CEA compared abrocitinib with standard of care (SoC) from a societal perspective, considering productivity losses. A hybrid decision tree and Markov model captured treatment phases, with inputs from clinical trials and real-world data.
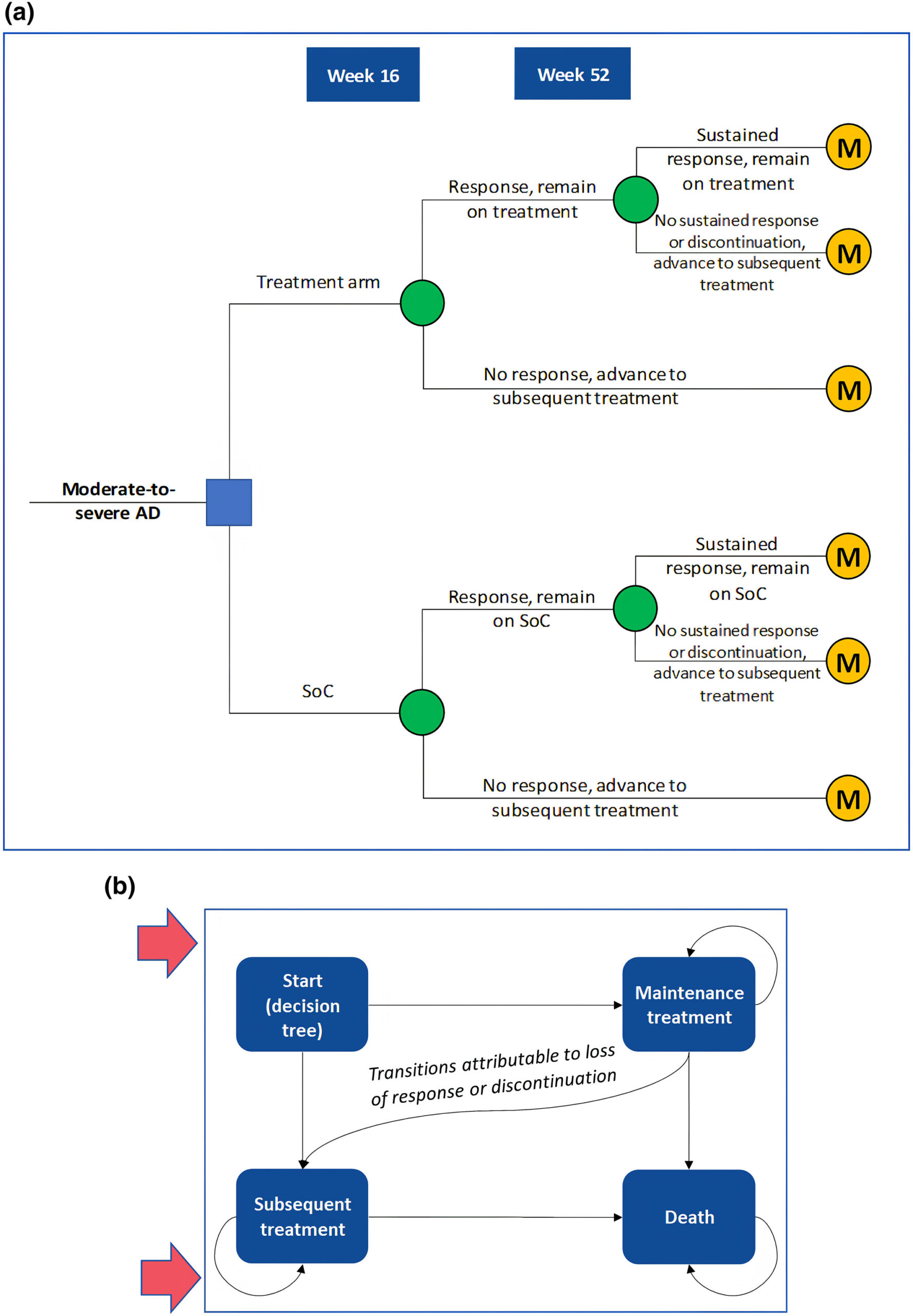
Analysis:
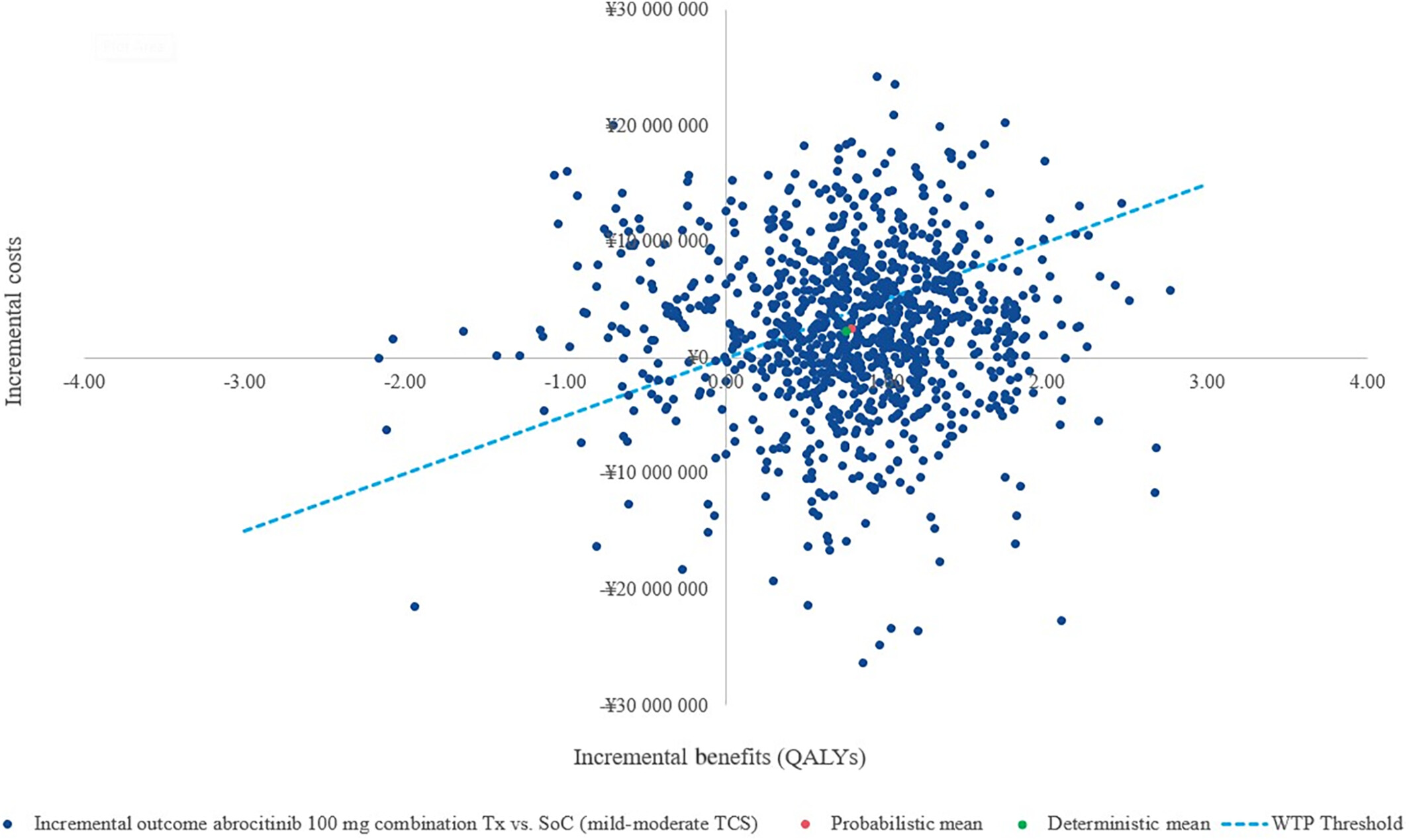
The analysis utilised a willingness-to-pay (WTP) threshold of ¥5,000,000 (approximately USD 38,023.4) per QALY. In evaluating the cost-effectiveness of abrocitinib compared to standard of care (SoC), a societal perspective revealed a gain of 0.75 quality-adjusted life years (QALYs) with an incremental cost-effectiveness ratio (ICER) of ¥3,034,514 per QALY. This perspective is crucial as it accounts for patient productivity loss, a significant factor in conditions like atopic dermatitis (AD). Sensitivity analysis highlighted the influence of response-based utility values and drug costs on the ICER in this societal viewpoint. The societal perspective considers healthcare costs and productivity, vital in evaluating interventions like abrocitinib for atopic dermatitis comprehensively.
Transitioning to a payer perspective, abrocitinib maintained its QALY gains but presented a higher ICER of ¥5,983,495 per QALY gained versus SoC. Under the payer lens, the emphasis shifted to utility weights and drug costs as pivotal factors affecting cost-effectiveness. The scenario analysis introduced a Japanese payer perspective, which differs from the societal viewpoint by concentrating solely on direct healthcare costs, omitting productivity loss factors.
Understanding these perspectives and their implications is essential for making informed decisions regarding healthcare resource allocation and treatment strategies.
Conclusion:
While future evaluations should incorporate emerging treatments such as dupilumab, this study establishes a crucial precedent by advocating for a societal viewpoint in cost-effectiveness assessments. Abrocitinib’s introduction offers a compelling, economically sustainable approach for treating moderate-to-severe atopic dermatitis, benefiting patients and Japan’s healthcare system. Lastly, both clinical efficacy and societal impact, this research underscores the importance of holistic viewpoints in evaluating novel AD therapies.
