
Introduction
Iliofemoral venous obstruction, arising from both thrombotic and non-thrombotic origins, significantly impacts patient well-being and imposes economic burdens. This condition includes acute deep vein thrombosis (aDVT), chronic post-thrombotic syndrome (PTS), May–Thurner syndrome, and non-thrombotic iliac vein lesions (NIVL), each requiring tailored treatment approaches. If left untreated, vascular diseases can lead to severe complications, including chronic pain, swelling, skin changes, and ulcerations. In more advanced cases, untreated venous obstructions, especially aDVT, can result in pulmonary embolism—a potentially life-threatening condition. Moreover, chronic venous insufficiency can significantly impair a patient’s quality of life, leading to reduced mobility and increased healthcare costs. Therefore, timely and effective intervention is crucial to prevent these adverse outcomes and improve patient well-being.
Current Treatment Strategies
Conservative management strategies for vascular diseases, such as the use of compression stockings, medication, and exercise therapies, are commonly employed to alleviate symptoms. However, these methods often fall short in providing complete relief, particularly in cases of significant venous obstruction. As a result, endovascular interventions, especially stent placement, have become increasingly popular among patients with symptomatic iliofemoral venous outflow obstruction. Stent placement offers a more direct and effective solution by physically opening up the obstructed veins, thereby improving blood flow and reducing symptoms.
Insights from the ABRE Study: Evaluating the Abre Venous Stent
To address these challenges, a self-expanding venous stent system, the Abre stent, was designed to treat symptomatic iliofemoral venous outflow obstruction. The ABRE study is a multicentre, investigational device exemption (IDE) prospective study. It provides crucial insights into the venous stent efficacy and safety. Black et al. evaluated the performance of the Abre stent in managing venous obstructions based on the three-year outcomes of the stent system.
Functional Outcomes and Quality of Life
Functional outcomes were assessed using various measures, including the Venous Insufficiency Epidemiological and Economics Study Quality of Life (VEINES-QoL/Sym), EQ-5D Index Score, Villalta score, and Revised Venous Clinical Severity Score (rVCSS). Significant improvements were observed at all follow-up points, 6-, 12-, 24-, and 36-months, compared to baseline. These improvements highlight the positive impact of the Abre stent on patients’ quality of life and symptom relief.
Long-Term Outcomes
The 36-month assessment revealed sustained primary (81.6%), primary-assisted (84.8%), and secondary patency (86.3%) rates, demonstrating the enduring effectiveness of the Abre stent. The study reported 100% device and lesion success, defined as the successful delivery of the device and venographic evidence of <50% residual stenosis post-dilation in the stented lesion, respectively. Significantly, the study reported a low incidence of major adverse events (MAEs), with the MAE rate at 36 months standing at 10.2%.
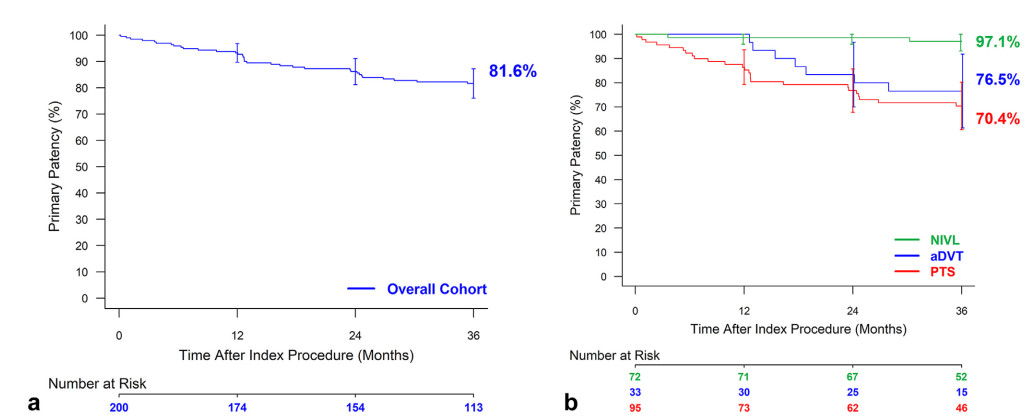
The results of sustained high patency and low MAE rates are promising as they lead to low levels of target lesion revascularisation and decreased possibility of reintervention. Thus, these findings highlight the stent’s safety profile and its impact on better patient outcomes over an extended period. The reduced need for reintervention and lower incidence of adverse events can lead to significant cost savings for healthcare systems, reducing the overall burden on medical resources and improving cost-effectiveness in the management of iliofemoral venous disease.
Conclusion
In conclusion, the intervention demonstrated in the ABRE study offers promising advancements in the treatment of iliofemoral venous obstruction, particularly for complex cases such as PTS. The statistically significant improvements in functional outcomes demonstrates the potential of this intervention to enhance the quality of life for patients suffering from chronic venous diseases. By providing a reliable and effective treatment option, this intervention can be particularly useful in specialised vascular centres and hospitals, where managing complex venous conditions is a priority. Furthermore, the positive long-term outcomes observed in the study suggest that widespread adoption of this intervention could lead to reduced healthcare costs associated with recurrent interventions and hospitalisations, ultimately contributing to a more efficient and effective healthcare system.
