
Introduction
Precision medicine stands as a guiding light in cancer care, tailoring treatments to tumour genetics. Next-Generation Sequencing (NGS) and Comprehensive Genomic Profiling (CGP) offer a significant advancement in personalised therapies, outperforming conventional treatments. However, the promise of precision medicine remains unmet without widespread access to essential biomarker testing. To ensure its effective implementation, a robust value framework is essential. A recent article, which has not yet been peer-reviewed, explores the development of a value framework for NGS in Europe, highlighting key criteria and stakeholder consensus.
Bridging the Gap: Value Assessment for Precision Medicine
Traditional Health Technology Assessments (HTAs) are inadequate in evaluating advanced diagnostics like NGS/CGP. These technologies, crucial for treatment decisions, demand a nuanced approach beyond conventional efficacy and safety parameters. The evolving nature of these tests post-approval poses unique regulatory and reimbursement challenges, necessitating adaptable assessment methods.
Despite endorsements from medical societies and initiatives like the EU Beating Cancer Plan, the adoption of NGS in Europe falls behind. Current practices analyse an insufficient percentage of necessary specimens, hindered by funding complexities and unclear value assessments. To fully realise the potential of NGS/CGP technologies, holistic Value Assessment Frameworks (VAFs) are mandatory.
Shaping the Future: A Collaborative Value Framework
A collaborative effort between the London School of Economics (LSE) and the Institute for Clinical Effectiveness and Health Policy (IECS) aimed to adapt a comprehensive VAF for NGS/CGP diagnostics in European oncology. This innovative framework, rooted in stakeholder insights, seeks to address the intricate value dimensions of these advanced diagnostic tools.
Exploring the preferences of diverse healthcare stakeholders, this research uncovers a strong consensus on key criteria, guiding the development of a novel co-created value framework. Addressing the fragmented adoption of NGS/CGP diagnostics in Europe, the study highlights the need for standardisation and equitable access across regions.
Final Framework and Key Findings
The final framework consisted of 23 ‘essential’ sub-criteria and four ‘complementary’ sub-criteria. Two sub-criteria were excluded due to not meeting the inclusion criteria. The framework’s development involved 81 European participants, with a 79% retention rate.
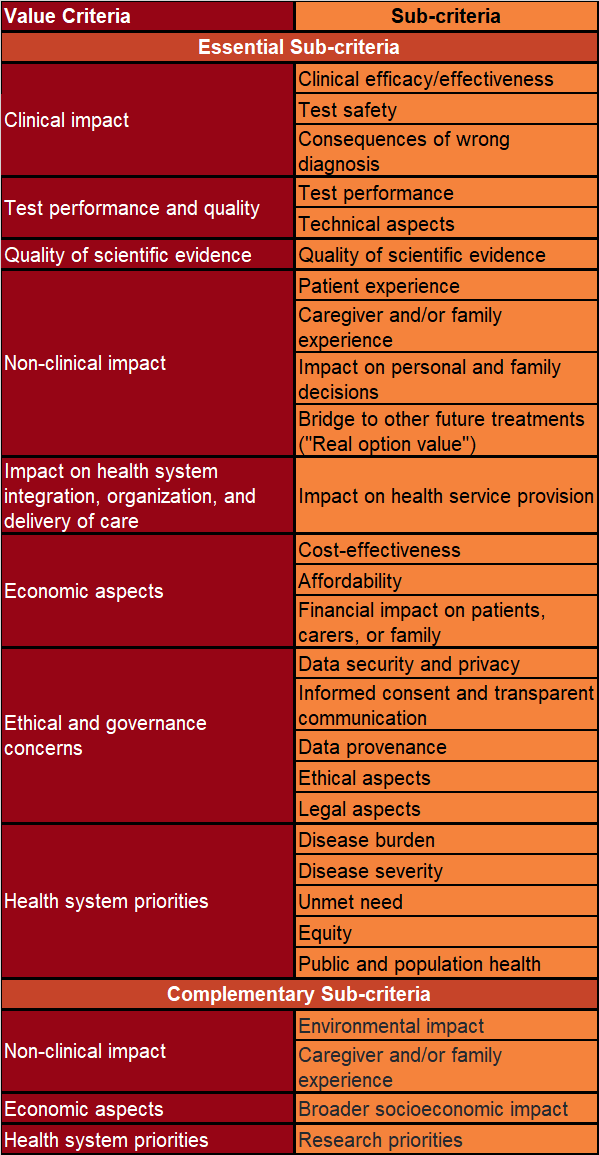
Embracing Change: Future Directions in Precision Medicine
As precision medicine evolves, the need for a standardised European framework for NGS/CGP value assessment becomes essential. Robust data infrastructure and outcomes-based financing models are critical to ensure equitable access and quality care in personalised medicine. By addressing data governance concerns and societal values, this value framework for NGS sets the stage for informed decision-making in oncology.
