Acute Heart Failure and its Impact
Acute Heart Failure (AHF) is a significant healthcare concern, affecting approximately six million people in the US. Projections suggest a dramatic increase in the cost of heart failure, from $3.7 billion in 2012 to $69.8 billion in 2030. The primary contributor to this increase is hospitalisation for AHF. Hospitalisation rates have been on the rise in the U.S, and mortality rates have been escalating since 2012. Moreover, lower socioeconomic status patients face a higher risk of hospitalisation and rehospitalisation in various conditions.
The Emergency Department (ED) and AHF
The Emergency Department (ED) is responsible for 70% to 80% of all AHF admissions in the U.S alone. A significant number of these admissions could potentially be avoidable. This is especially true for lower-risk patients. Hospitalising these patients could potentially expose them to unnecessary in-hospital adverse events and affect their quality of life.
Short-Stay Units (SSUs): A Potential Solution
Short-Stay Units (SSUs), offering brief periods of observation (<24 hours), have been supported for lower-risk patients with AHF. Prior studies have been conducted and have shown a shorter length of stay in SSU patients compared with inpatients. However, these studies were limited by their nonrandomised design.
The SSU-AHF Trial
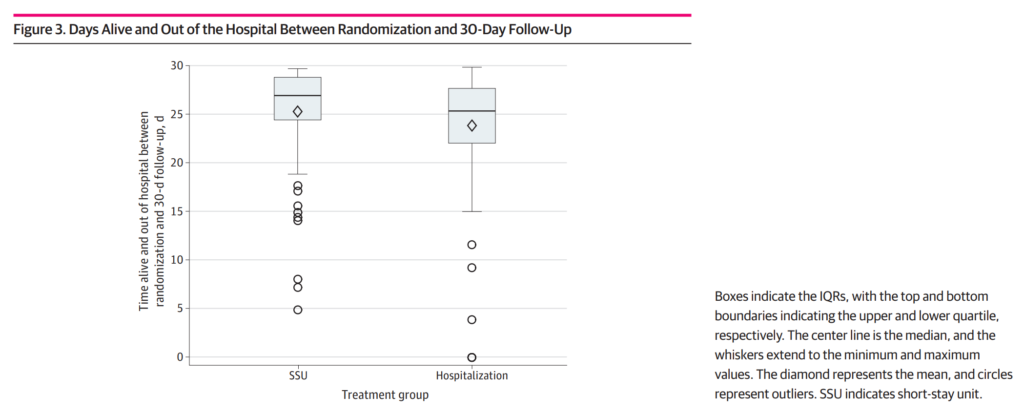
The SSU-AHF trial was designed to overcome some of the previous limitations and evaluate outcomes in a randomised trial setting. The trial found no significant differences between SSU treatment and hospitalisation in terms of KCCQ-12 scores (a measure of quality of life) at day 3. However, the number of Days Alive Out Of Hospital (DAOOH) at 30 days significantly increased in the SSU group.
Implications of the SSU-AHF Trial
The SSU-AHF trial suggests that SSUs offer a safe alternative setting for trying novel approaches to risk stratification, ongoing treatment, and rigorous self-care assessment. While the study did not show a significant benefit of SSU over hospitalisation, it does however support the consideration of SSU management instead of routine hospital admission. This would potentially assist with overcoming some of the increased cost expected to arise in the near future.
Limitations and Conclusions
The SSU-AHF trial had some limitations, including a relatively small sample size and the impact of the COVID-19 pandemic on study parameters. We need more randomised clinical trial data to definitively test the SSU strategy’s benefits.
