The Importance of Developing a Brand-New Structure
The field of digital health technologies (DHTs), which offers novel solutions for the delivery of healthcare, is undergoing rapid development. However, doing an evaluation of their usefulness presents a novel obstacle due to the fact that conventional frameworks for health technology such as pharmaceuticals and medical equipment cannot be directly applied to them. The Peterson Health Technology Institute (PHTI) has established a value assessment framework to guide evaluations of digital health technologies (DHTs), with the goals of creating evidentiary standards for technology developers and assisting organisations in adopting impactful DHTs. This methodology is illustrated below.
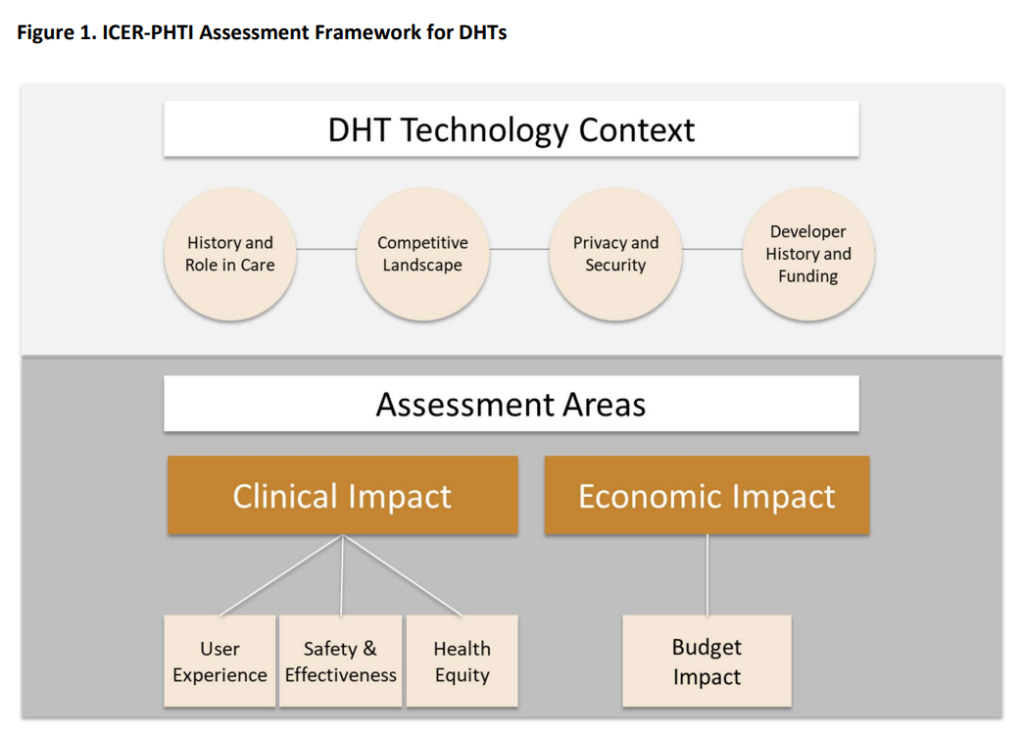
The Framework of the Peterson Health Technology Institute
The evaluation of the value of DHTs must pay close attention to the ways in which they affect the economy. DHTs are projected to improve care outcomes while simultaneously reducing overall costs, or give dramatically improved access at significantly cheaper costs than other options. This is in contrast to new medications, which often increase the cost to the health system. Because of the one-of-a-kind characteristics of DHTs, the PHTI paradigm places a greater emphasis on cost-effectiveness analysis over long-term cost-benefit analysis.
The methodology also takes into account the larger economic benefits of DHTs, such as increases in productivity as well as decreases in the amount of time as well as money spent by families and caregivers. However, these advantages will not be considered in the major study of how the budget would be impacted.
Digital Health Technologies
The PHTI’s approach for evaluating DHTs is a significant step towards standardising the evaluation of these technologies and marks an important step in that direction. The framework will guide technology developers in their product development by defining clear and thorough criteria of evidence, and it will assist healthcare organisations in making educated decisions about using DHTs.
The PHTI’s framework will, in the end, contribute to improved health outcomes as well as cost savings in the healthcare sector. This will be accomplished by guaranteeing that only the DHTs that are the most effective and cost-efficient will be adopted.