Introduction
Migraine is a complex neurological condition marked by moderate to severe headaches that can last for hours to days, with lingering symptoms and extreme tiredness post-attack. The International Headache Society defines chronic migraine, the most severe form, as having headaches on 15 or more days per month for over three months, with migraine characteristics on at least 8 days per month. Chronic migraine affects 10-20% of migraine sufferers and significantly impacts health-related quality of life of the patient and their families.
Migraine is highly prevalent, with over 200 000 patients in the Netherlands. It is more common in women, particularly during their childbearing and working years. Globally, migraines contribute significantly to disability, being the 14th leading cause of disability-adjusted life-years in 2019 and the top cause of years lived with disability for those aged 15-49. This results in a substantial economic burden, with costs in the Netherlands estimated between €2.3–4.2 billion annually, potentially reaching up to €5 billion.
There are preventative treatments for migraine, one of the newest drugs being fremanezumab, a monoclonal antibody designed to prevent migraines. It works by targeting and inhibiting the calcitonin gene-related peptide (CGRP), a molecule involved in migraine attacks. Clinical trials have shown that fremanezumab significantly reduces the frequency of monthly migraine days (MMD), improving patients’ quality of life.
Fremanezumab vs. Supportive Care
This Dutch study published in BMC Neurology explores the cost-effectiveness of fremanezumab, comparing it with best supportive care (BSC) and examining its long-term benefits. The primary patient population included those with chronic migraine who did not respond to topiramate, valproate, or onabotulinumtoxinA. In the Netherlands, healthcare providers consider fremanezumab a last-line treatment for these patients. The model began with a decision tree to evaluate treatment response after 12 weeks. Responders continued treatment, while non-responders discontinued. Patients then entered a semi-Markov model with two health states (on treatment; off treatment) and an absorbing state of death (natural causes). Within the main health states, patients were distributed across 29 monthly migraine days. The model structure is illustrated in Figure 1 below.
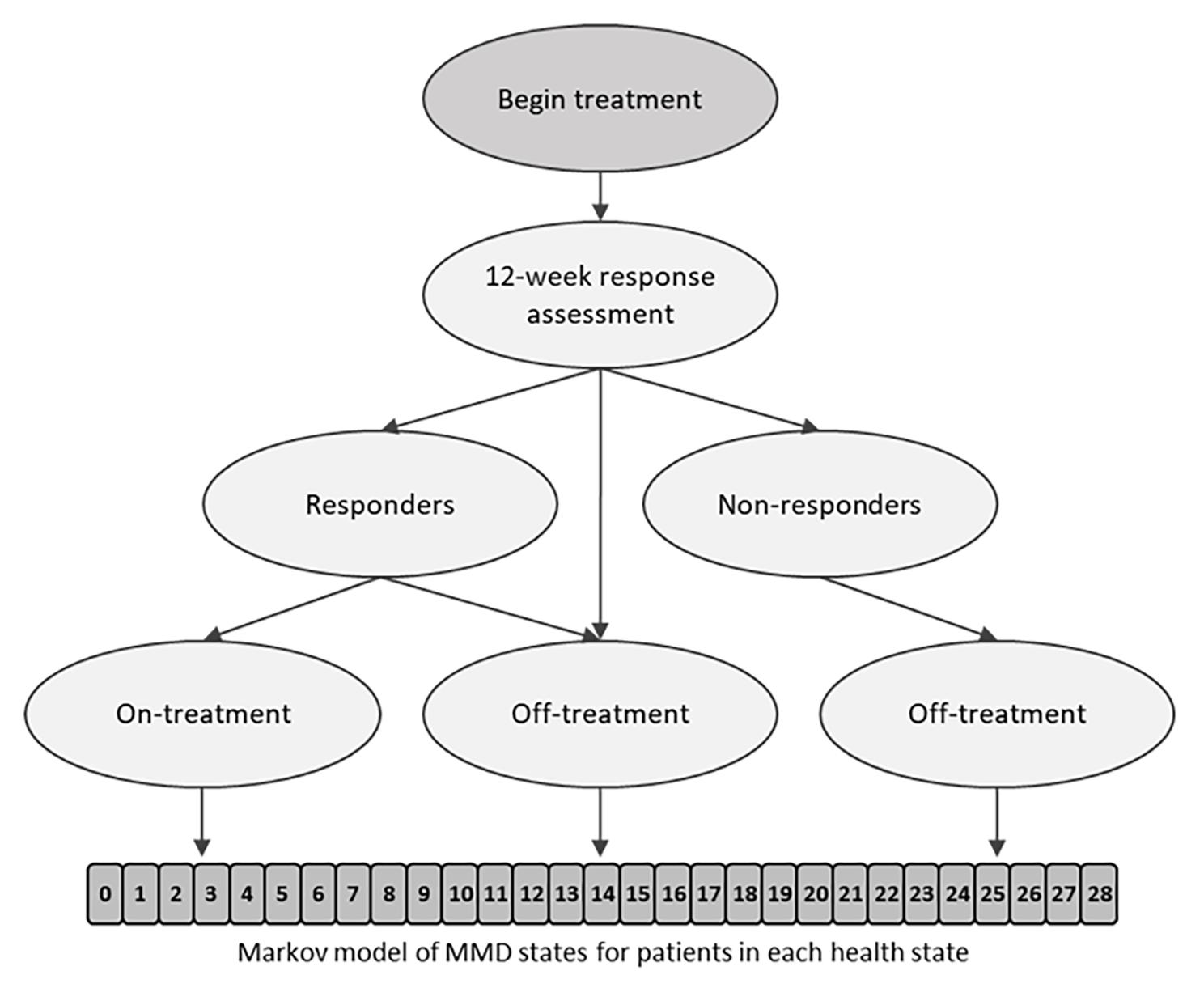
Figure 1. Model structure. A responder was a patient with a ≥ 30% reduction in MMD from baseline during the 12 weeks after therapy initiation. MMD, monthly migraine days.
Economic Evaluation
The researchers performed the study from a societal perspective and included an economic evaluation of fremanezumab involving direct and indirect costs. Direct costs include medical expenses such as hospitalisation, emergency department visits, and medication. Indirect costs encompass productivity losses due to missed workdays and the need for informal care.
Cost-Effectiveness Analysis
A cost-effectiveness analysis compares the costs and health outcomes of fremanezumab with BSC. The primary measure used is the incremental cost-effectiveness ratio (ICER), which represents the cost per quality-adjusted life year (QALY) gained. An ICER below the willingness-to-pay (WTP) threshold indicates that the treatment is cost-effective.
Key Findings
Costs Compared to Supportive Care: In the primary study population, fremanezumab is cost-saving compared with supportive care, saving €2 514.
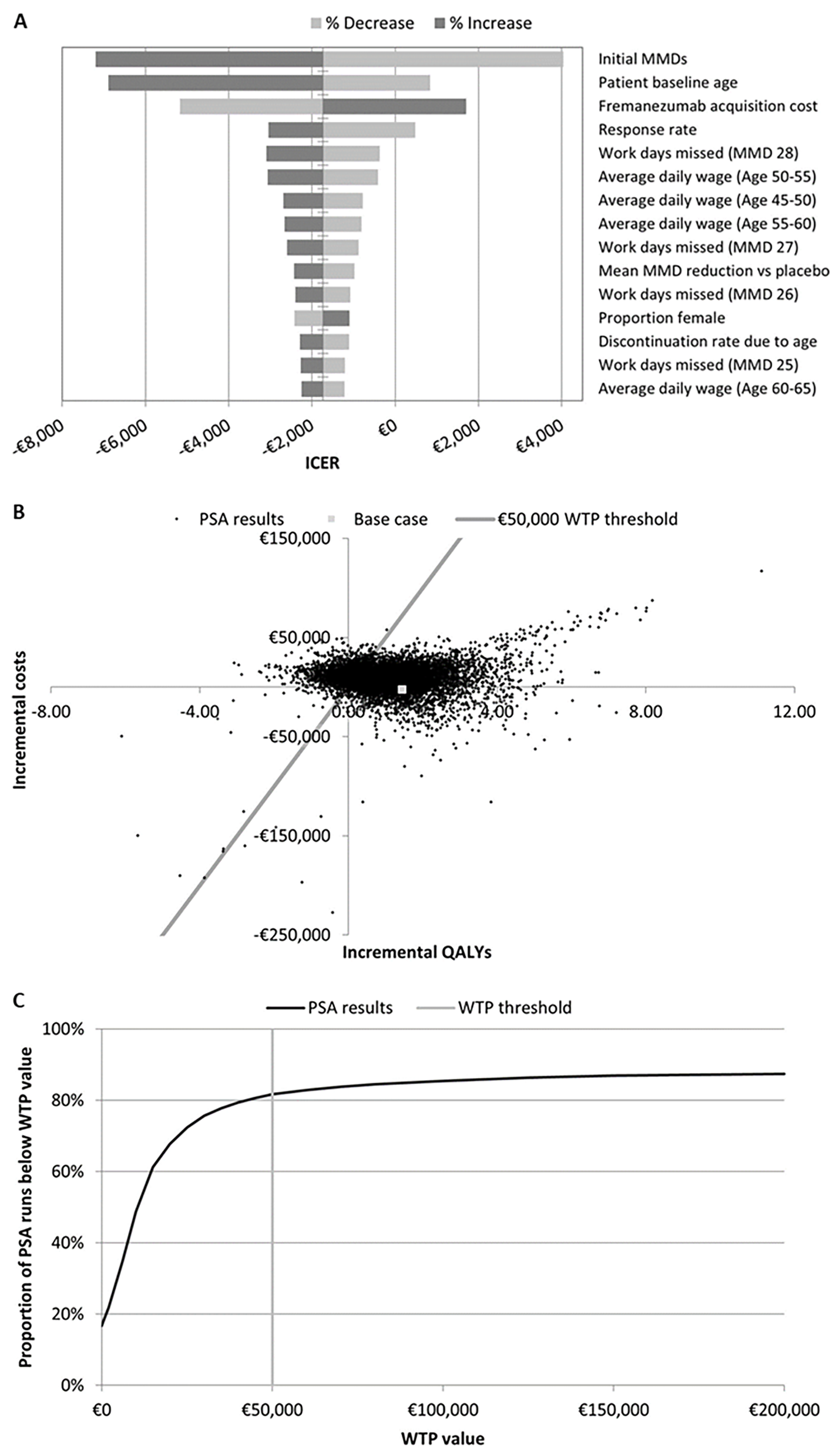
Long-Term Benefits: Over a 10-year time horizon, fremanezumab remained cost-effective, with an ICER of €4 620 per QALY. This is well below the WTP threshold of €50 000, demonstrating its sustained economic value over time.
Indirect Costs: Including indirect costs generally made fremanezumab dominant, meaning it was both more effective and less costly than BSC. When productivity losses are excluded, the ICER for fremanezumab increases significantly to €17 498 per QALY. Despite this increase, the ICER remains within the range considered cost-effective. Moreover, including informal care costs also results in substantial cost savings.
Most Impactful Variables: Monthly migraine days, starting age of treatment, fremanezumab cost, and response rate were the most impactful variables on the model outcomes. Fremanezumab consistently proved its high cost-effectiveness compared to supportive care.
Figure 2. A) Tornado plot of sensitivity analysis results. B and C, respectively) scatter plot of and WTP curve for PSA results. ICER, incremental cost-effectiveness ratio; MMD, monthly migraine days; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; WTP, willingness-to-pay.
Conclusion
This paper presents the first published cost-effectiveness study for fremanezumab, demonstrating its substantial economic impact in migraine management. It offers a cost-effective solution for reducing the burden of migraines, improving patient outcomes, and lowering overall healthcare costs. As healthcare systems continue to seek value-based treatments, fremanezumab stands out as a promising option.