
Introduction
Health state valuation (HSV) is a crucial aspect of healthcare assessments. In traditional practice, valuations have historically anchored around ‘dead’. However, recent discussions suggest that this practice may be unnecessary and problematic. There might be implications of removing ‘dead’ from HSV but it proposes new approaches for future research.
The Problem with ‘Dead’ as an Anchor
Anchoring HSV at ‘dead’ has been a standard practice. However, this approach has several issues. Firstly, it introduces bias into people’s health state preferences. When ‘dead’ is used as a reference point, it can skew the valuation of health states. This bias affects all HSVs and can lead to inaccurate assessments.
Moreover, the distinction between states better or worse than dead (BTD and WTD) is problematic. These terms hold significant meaning outside of HSV, such as in discussions about physician-assisted dying. Removing ‘dead’ from HSV allows for more precise and meaningful usage of these terms.
Implications for Health State Valuation
Removing ‘dead’ from HSV changes how we should discuss and interpret these valuations. Firstly, new approaches to valuation are needed. Excluding ‘dead’ does not solve the challenge of calculating HSVs for health states that generate very high shortfalls (VHY) greater than one. Traditional time trade-off (TTO) methods cannot offer shorter time periods in full health to generate such shortfalls. Hence, we must explore alternative methods.
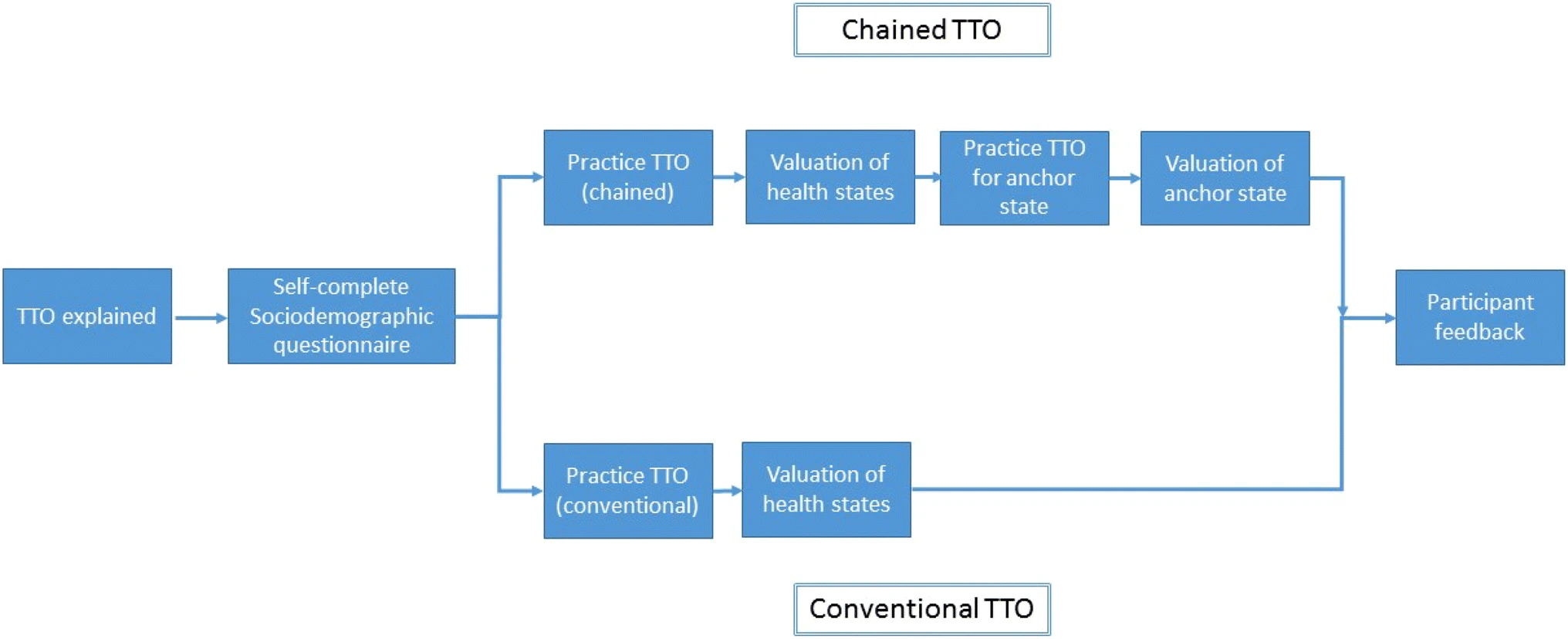
Exploring New Valuation Methods
One potential approach is the use of chained TTO. This method involves a sequence of periods, some generating VHY shortfalls due to poor health and others generating quality-adjusted life years (QALYs) in full health at non-concurrent times. Full health may occur before or after ill-health, known as ‘lead time’ or ‘lag time’. Another option is to identify shortfalls relative to a hypothetical life expectancy. These methods must ensure plausible scenarios for respondents and account for time preference.
Furthermore, one can derive insights from other methodologies, like engaging in kaizen tasks. These tasks involve continuous improvement and can provide valuable insights into new valuation methods.
Assessing the Importance of Negative States
In light of this reinterpretation, it is essential to assess the importance of negative states. The frequency of states experiencing a shortfall greater than one VHY, how often practitioners observe these states in practice, and the relevance of these states to health technology assessment remain unknown. Understanding these characteristics is crucial for accurately modelling negative values.
Most valuation studies have used the EQ-5D as an example. However, this analysis applies to any patient-reported outcome measure or set of HSVs used to estimate QALYs. Negative HSVs can offer valuable insights into health states, but individuals should avoid labeling them as worse than dead (WTD). Researchers should abandon the search for a state with an HSV equal to zero. QALYs represent the value of a healthy year, which has no meaningful relation to the value of dying or being dead.
Conclusion
The practice of anchoring HSV at ‘dead’ is outdated and introduces bias into health state valuations. Removing ‘dead’ from HSV allows for more accurate and meaningful assessments. Future research should focus on developing new valuation methods and understanding the importance of negative states. By doing so, we can improve the accuracy and relevance of health state valuations in healthcare assessments.
