
Introduction
There are plans to implement Health Technology Assessment in South Africa on a wide scale. This will make sure that all HTA processes are standardized with the aim of using them for prioritizing areas where funds need to be allocated thus achieving fairness and effectiveness at the same time under National Health Insurance system. Although there have been some initiatives already carrying out HTAs, they lack continuity. By establishing a Ministerial Advisory Committee for HTA, the country hopes that creation of an independent and strong entity will assist on advising decision makers in the health sector.
The Development of Health Technology Assessment in South Africa
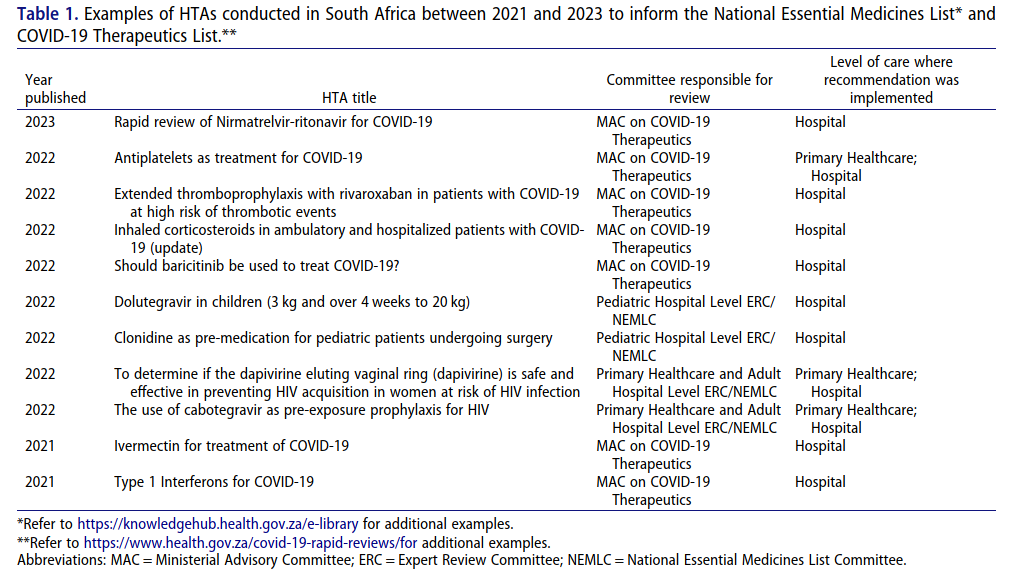
HTA has evolved in South Africa mainly through selection of Essential Medicines for the NEML (National Essential Medicine List). The latter guides procurement within public institutions and public health programs rely heavily on it. However different bodies perform HTA functions leading to duplication hence inefficiency. The NEML Committee historically concentrated much on evaluating drugs without adequate resources allocated towards this end. Additional efforts are also needed to strengthen the evidence-based approach to broadened the scope to include various medical devices and procedures.
The Significance of HTA under NHI
According to the NHI design, there should be a framework for HTA which starts at ministerial level before expanding into a national entity. This new body shall use scientific methods when assessing different healthcare interventions so that resources can be fairly distributed among them. The passing into law of the NHI Bill in 2024 marks significant progress. The post approval phase will involve HTAs being used to define a cost effective benefit package as well as shaping strategic purchasing decisions. It is also planned that this model should start off small but expand over time to ensure it’s sustainable implementation.
Strategies for Effective Implementation
For HTA to become a fixed part of the way things are done, current systems must be utilized. The first step is to create the Ministerial Advisory Committee and establish HTA policy frameworks. Secondly, contracting and involving stakeholders will be used to strengthen national HTA resources in the medium term. In the long run, the NHI should operationalize HTA processes to a level where they may become an independent body. This will mean investment in human resource capacity building, governance structures as well collaborative relationships with academic institutions.
Conclusion
Using HTA systematically under the NHI could lead to more efficient investment in healthcare. Clear methods for decision-making should be used when resource allocation decisions are involved. The successful incorporation of HTA into the NHI framework will depend on strong leadership, stakeholder collaboration, and ongoing commitment to capacity building. Ensuring equitable access to quality healthcare for all South Africans will ultimately validate the efforts to institutionalize HTA.
