The Purpose and Pricing of Efgartigimod Alfa
Efgartigimod Alfa, a drug designed to ameliorate symptoms of Generalised Myasthenia Gravis (gMG), has been reimbursed since April 2023 in Japan. The drug is priced at JPY 421,455 for Vyvgart® for intravenous infusion 400 mg as of September 2023. Center for Outcomes Research and Economic Evaluation for Health (C2H) calculate the price using a model for the Efgartigimod Alfa Cost-effectiveness. They then add a 5% usefulness premium and a 10% market premium.
The Scope of the Efgartigimod Alfa Cost-effectiveness
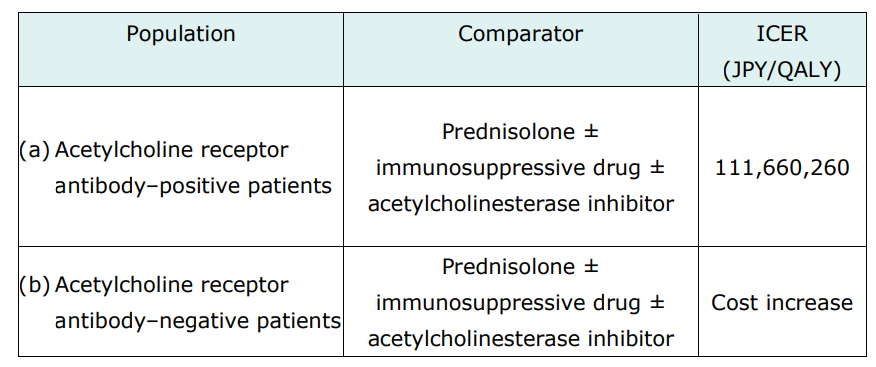
The cost-effectiveness evaluation of Efgartigimod alfa targets two populations: acetylcholine receptor antibody-positive and antibody-negative patients. The comparators were prednisolone ± immunosuppressive drug ± acetylcholinesterase inhibitor.
Evaluation of Additional Benefits
The manufacturer did not conduct a systematic review in the base case analysis but evaluated the additional benefits of Efgartigimod alfa for patients with gMG as a whole. The academic group decided to evaluate based on scope. They assessed additional benefits for each population using the ADAPT trial.
Results of the Cost-effectiveness Analysis
The manufacturer conducted a cost-utility analysis using a Markov model, which had states of Efgartigimod alfa or the comparator as first-line treatments, intravenous immunoglobulin or plasma exchange as second-line treatments, eculizumab as third-line treatment (only for population [a]), and the best supportive care.
The Expert Committee of Cost-Effectiveness Evaluation accepted the following: