The Promise and Challenges of Gene Therapies
Gene therapies offer transformational benefits to patients, health systems, and society. However, several challenges hinder timely patient access. A report commissioned by Pfizer, penned by Besley et al. (2022), discusses the difficulties of health technology assessment (HTA) of gene therapies and proposes potential solutions.
Gene therapies, as defined by the European Medicines Agency (EMA), are medicines containing genes that lead to therapeutic, prophylactic, or diagnostic effects. They work by inserting ‘recombinant’ genes into the body to treat a variety of diseases, including genetic disorders, cancer, or long-term diseases.
Recommendations for Overcoming HTA Challenges
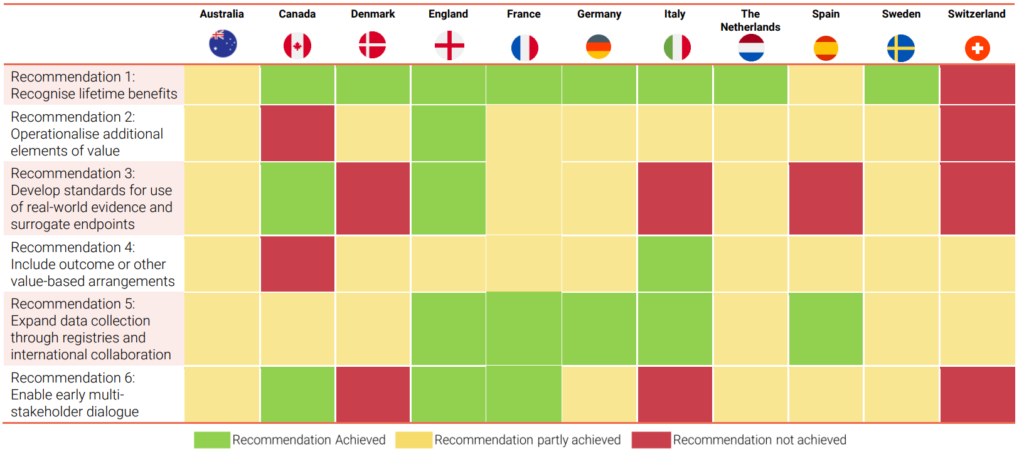
Based on a review of the literature and insights from an international panel of HTA and health economics experts, the authors propose six overarching recommendations. These recommendations aim to address the challenges in fully capturing the potential value of gene therapies as part of the HTA process and to improve the quality and acceptability of the evidence generated.

The recommendations are not specific to gene therapies and should be consistently applied across HTA of other treatments. However, due to the unique challenges presented by the HTA of gene therapies, if implemented, these recommendations are likely to have a larger impact on the assessment of gene therapies.
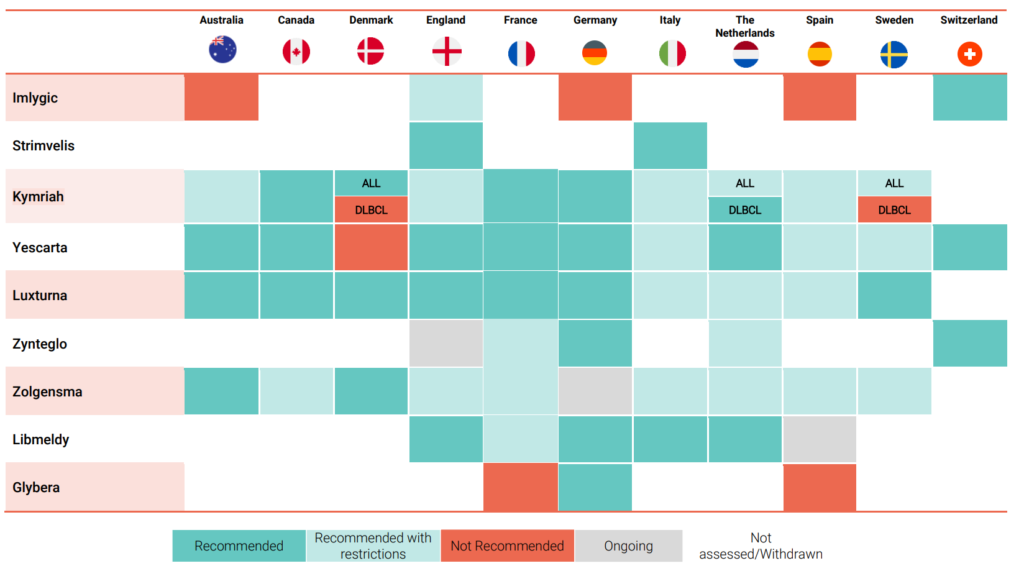
The report also assesses the extent to which these recommendations are being achieved in selected European countries, Australia, and Canada. It reviews the HTA outcomes of key gene therapies conducted in these countries to provide further understanding of the current state of play.