
A New Class IA Recommendation for Heart Failure Treatment
Heart failure, a major cause of hospital admissions in the U.S., comes with high morbidity, mortality, and financial costs. Recent advances in pharmacotherapy for heart failure with reduced ejection fraction (HFrEF) have given rise to new disease-modifying therapies. One such novel angiotensin receptor neprilysin inhibitor (ARNI) is sacubitril-valsartan.
The PARADIGM-HF clinical trial found that sacubitril-valsartan reduced the risk of cardiovascular death or hospitalization due to heart failure (HF), among ambulatory patients with an HFrEF of 40% or less. This makes it a highly cost-effective treatment option.
Broadening the Scope of Sacubitril-Valsartan
In February 2021, the U.S. Food and Drug Administration expanded the labelling of sacubitril-valsartan to include all adult patients with chronic HF. This decision was based on the understanding that the benefits are most evident in patients with left ventricular ejection fraction (LVEF) below normal. However, the upper bound of below-normal EF is not well defined.
A recent economic evaluation of treatment with sacubitril-valsartan vs renin-angiotensin system inhibitors (RASis) in patients with HF across a broader range of EFs found the treatment to be of high economic value for patients with EF≤50%. It showed intermediate value up to an EF of 60% or less before accounting for any price concessions that payers may receive.
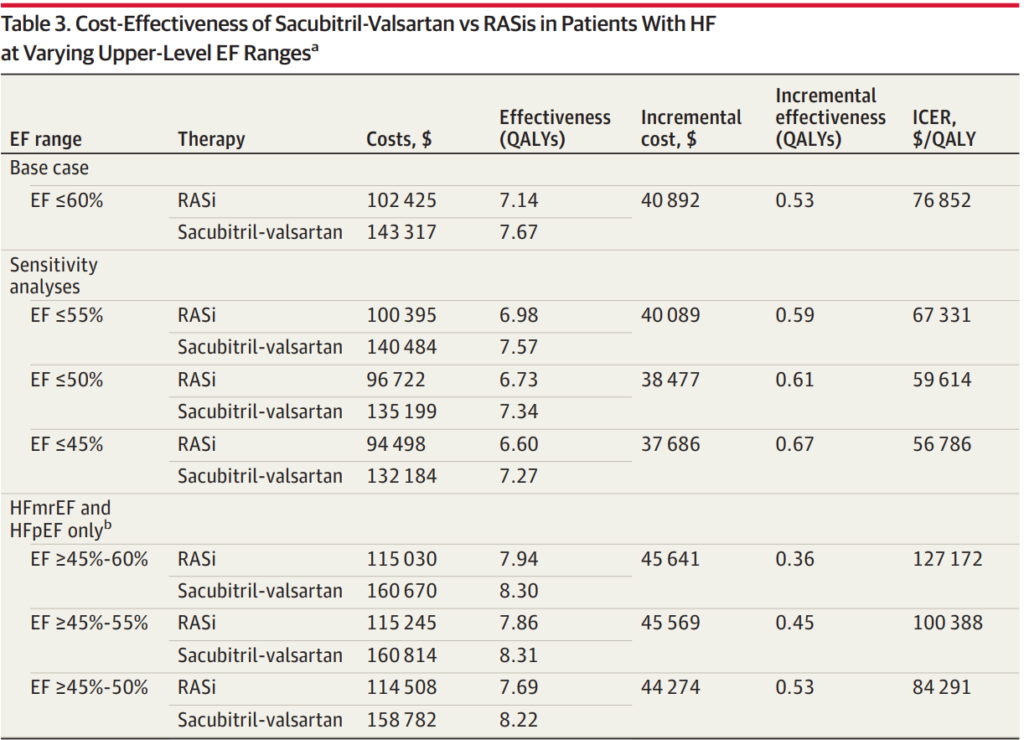
Commentary from Cohen at al., mentioned that the economic value of sacubitril-valsartan is comparable to sodium-glucose cotransporter 2 inhibitors (SGLT2is). Both are dependent on drug price. SGLT2is were estimated to be intermediate value costing $126 500 and $139 000 per quality-adjusted life year in those with LVEFs of 40% to 49% and 50% to 59%, respectively. However, these analyses do not consider the affordability of the medications for individuals. Economic assessments of these novel medications suggest they could add billions to US healthcare spending. Without efforts to lower drug prices, the use of costly, intermediate- to low-value care may be reinforced.
Conclusion
Sacubitril-valsartan increases quality-adjusted life years (QALYs) at an incremental cost-effectiveness ratio consistent with high economic value for patients inclusive of reduced and mildly reduced EFs (≤50%). The data analysis of 13,264 total patients showed that for those with EFs of 60% or less, sacubitril-valsartan could potentially add 0.53 quality-adjusted life-years (QALYs). This would come at an incremental lifetime cost of $40,892 compared to RASi, resulting in an ICER of $76,852 per QALY. Therefore, it is estimated to be of at least intermediate value when using the current undiscounted wholesale acquisition cost price across a broad HF population with EFs below normal, when below normal is defined as an EF of 60% or less.
